ImmunoPET of tissue factor expression in triple-negative breast cancer with a radiolabeled antibody Fab fragment
- PMID: 25801992
- PMCID: PMC4482783
- DOI: 10.1007/s00259-015-3038-1
ImmunoPET of tissue factor expression in triple-negative breast cancer with a radiolabeled antibody Fab fragment
Abstract
Purpose: To date, there is no effective therapy for triple-negative breast cancer (TNBC), which has a dismal clinical outcome. Upregulation of tissue factor (TF) expression leads to increased patient morbidity and mortality in many solid tumor types, including TNBC. Our goal was to employ the Fab fragment of ALT-836, a chimeric anti-human TF mAb, for PET imaging of TNBC, which can be used to guide future TNBC therapy.
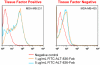
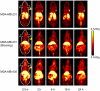
Methods: ALT-836-Fab was generated by enzymatic papain digestion. SDS-PAGE and FACS studies were performed to evaluate the integrity and TF binding affinity of ALT-836-Fab before NOTA conjugation and (64)Cu-labeling. Serial PET imaging and biodistribution studies were carried out to evaluate the tumor targeting efficacy and pharmacokinetics in the MDA-MB-231 TNBC model, which expresses high levels of TF on the tumor cells. Blocking studies, histological assessment, as well as RT-PCR were performed to confirm TF specificity of (64)Cu-NOTA-ALT-836-Fab.
Results: ALT-836-Fab was produced with high purity, which exhibited superb TF binding affinity and specificity. Serial PET imaging revealed rapid and persistent tumor uptake of (64)Cu-NOTA-ALT-836-Fab (5.1 ± 0.5 %ID/g at 24 h post-injection; n = 4) and high tumor/muscle ratio (7.0 ± 1.2 at 24 h post-injection; n = 4), several-fold higher than that of the blocking group and tumor models that do not express significant level of TF, which was confirmed by biodistribution studies. TF specificity of the tracer was also validated by histology and RT-PCR.
Conclusion: (64)Cu-NOTA-ALT-836-Fab exhibited prominent tissue factor targeting efficiency in MDA-MB-231 TNBC model. The use of a Fab fragment led to fast tumor uptake and good tissue/muscle ratio, which may be translated into same-day immunoPET imaging in the clinical setting to improve TNBC patient management.
Figures
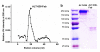



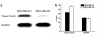
References
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Engl J Med. 2010;363:1938–48. - PubMed
-
- Terwisscha van Scheltinga AG, Berghuis P, Nienhuis HH, Timmer-Bosscha H, Pot L, Gaykema SB, et al. Visualising dual downregulation of insulin-like growth factor receptor-1 and vascular endothelial growth factor-A by heat shock protein 90 inhibition effect in triple negative breast cancer. Eur J Cancer. 2014;50:2508–16. - PubMed
-
- Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. - PubMed
-
- Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–201. - PubMed
-
- Vrana JA, Stang MT, Grande JP, Getz MJ. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor beta family. Cancer Res. 1996;56:5063–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

