Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer
- PMID: 25802102
- PMCID: PMC4393805
- DOI: 10.1007/s10585-015-9714-5
Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer
Abstract
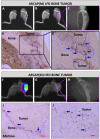
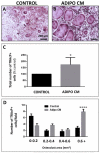
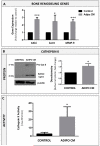
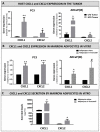
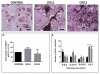
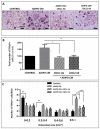
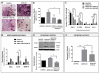
Increased bone marrow adiposity is a common feature of advanced age, obesity and associated metabolic pathologies. Augmented numbers of marrow adipocytes positively correlate with dysregulated bone remodeling, also a well-established complication of metastatic disease. We have shown previously that marrow adiposity accelerates prostate tumor progression in the skeleton and promotes extensive destruction of the bone; however, the factors behind adipocyte-driven osteolysis in the skeletal tumor microenvironment are not currently known. In this study, utilizing in vivo diet-induced models of bone marrow adiposity, we reveal evidence for positive correlation between increased marrow fat content, bone degradation by ARCaP(M) and PC3 prostate tumors, and augmented levels of host-derived CXCL1 and CXCL2, ligands of CXCR2 receptor. We show by in vitro osteoclastogenesis assays that media conditioned by bone marrow adipocytes is a significant source of CXCL1 and CXCL2 proteins. We also demonstrate that both the adipocyte-conditioned media and the recombinant CXCL1 and CXCL2 ligands efficiently accelerate osteoclast maturation, a process that can be blocked by neutralizing antibodies to each of the chemokines. We further confirm the contribution of CXCR2 signaling axis to adiposity-driven osteoclastogenesis by blocking fat cell-induced osteoclast differentiation with CXCR2 antagonist or neutralizing antibodies. Together, our results link CXCL1 and CXCL2 chemokines with bone marrow adiposity and implicate CXCR2 signaling in promoting effects of marrow fat on progression of skeletal tumors in bone.
Figures
References
-
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nature clinical practice. 2006;2(1):35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

