A highly sensitive novel immunoassay specifically detects low levels of soluble Aβ oligomers in human cerebrospinal fluid
- PMID: 25802556
- PMCID: PMC4369838
- DOI: 10.1186/s13195-015-0100-y
A highly sensitive novel immunoassay specifically detects low levels of soluble Aβ oligomers in human cerebrospinal fluid
Abstract
Introduction: Amyloid β-protein oligomers play a key role in Alzheimer's disease (AD), but well-validated assays that routinely detect them in cerebrospinal fluid (CSF) are just emerging. We sought to confirm and extend a recent study using the Singulex Erenna platform that reported increased mean CSF oligomer levels in AD.
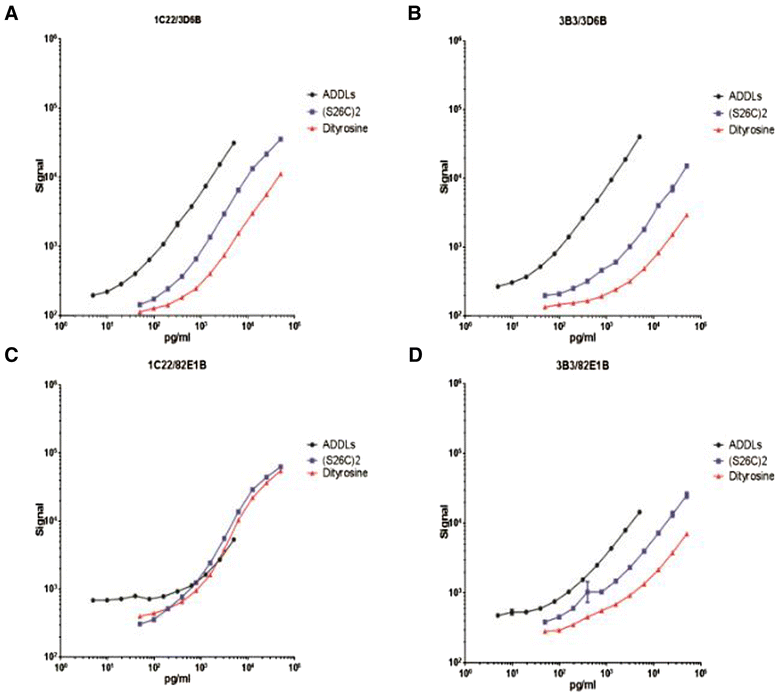
Methods: We tested four antibody pairs and chose one pair that was particularly sensitive, using 1C22, our new oligomer-selective monoclonal antibody, for capture. We applied this new assay to extracts of human brain and CSF.
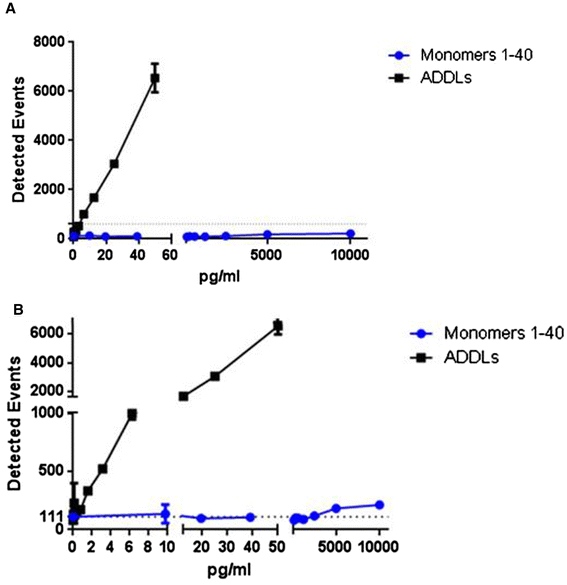
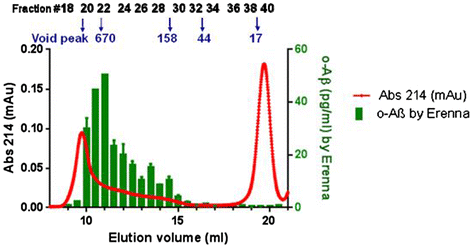
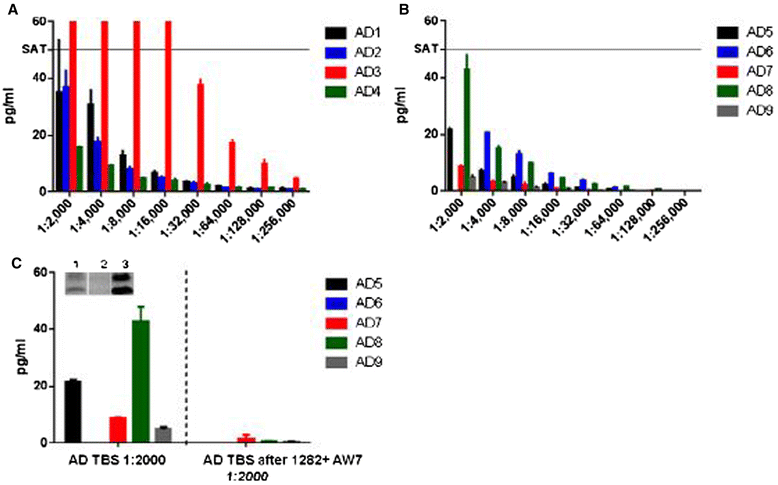
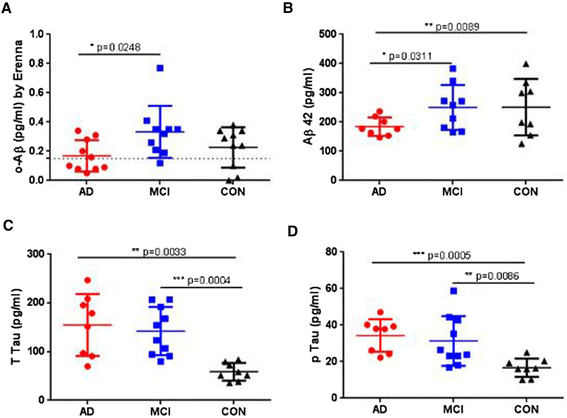
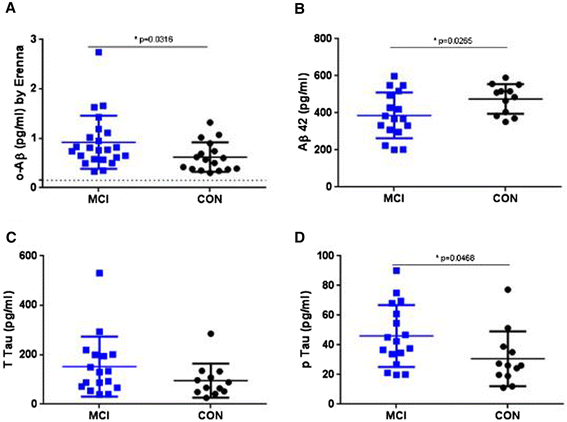
Results: A combination of 1C22 for capture and 3D6 for detection yielded an Erenna immunoassay with a lower limit of quantification of approximately 0.15 pg/ml that was highly selective for oligomers over monomers and detected a wide size-range of oligomers. Most CSFs we tested had detectable oligomer levels but with a large overlap between AD and controls and a trend for higher mean levels in mild cognitive impairment (MCI) than controls.
Conclusion: Aβ oligomers are detectable in most human CSFs, but AD and controls overlap. MCI CSFs may have a modest elevation in mean value by this assay.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

