Silicon Matrix Calcium Phosphate as a Bone Substitute: Early Clinical and Radiological Results in a Prospective Study With 12-Month Follow-up
- PMID: 25802604
- PMCID: PMC4365826
- DOI: 10.1016/SASJ-2007-0122-RR
Silicon Matrix Calcium Phosphate as a Bone Substitute: Early Clinical and Radiological Results in a Prospective Study With 12-Month Follow-up
Abstract
Introduction: Autograft has been the "gold standard" for orthopedic bone grafting applications, but with some clinical challenges. Here we present the rationale and clinical outcomes supporting the use of a bone substitute material that consists of a mixture of two calcium phosphates (HA and ß-TCP), which are integrated into a silicon xerogel matrix, promoting nanocrystalline apatite layers on the surface of the material following implantation into a physiological environment.
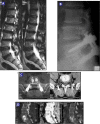
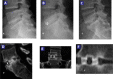
Methods: Twenty-four patients with a median age of 53.80 (36-81) years underwent lumbar spinal fusion for degenerative disease, selected by clinical presentation, X-rays, and MRI findings. Subjects were evaluated preoperatively and postoperatively at 1, 3, 6, and 12 months. The outcome assessment consisted of visual analog scale (VAS), Oswestry Disability Index (ODI), and radiological assessment analyzing the state of fusion on X-ray and CT evaluation by 3 independent radiologists.
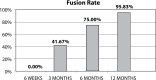
Results: All patients completed 12-month follow-up. The mean VAS decreased from 9.3 (± 0.9) to 2.4 (± 1.6) and the mean ODI decreased from 55.0 (± 9.2) to 19.3 (± 11.4) at 12-month follow-up. Three months after surgery, 10 patients (41.67%) had solid fusion based on analysis of CT scans and dynamic radiographs. At 6 months postoperatively, the fusion rate had increased to 75% (18 patients). Twelve months after surgery, 95.83% of patients had solid fusion (23 patients).
Conclusions: The clinical results from this study of silicon matrix calcium phosphate are consistent with previous in vitro studies indicating that this material stimulates formation of a bioactive layer and provides an effective bone graft material for lumbar fusion applications. In comparison with previous studies involving rhBMP-2, silicon matrix calcium phosphate provided a lower fusion rate at 3- and 6-month follow-up points, but after 12 months, the fusion rate was similar, with no statistical differences and lower overall costs. No clinically relevant adverse events were associated with either the cage or graft material. With increasing evidence of high rates of enhanced fusion development in this spinal application, additional research is encouraged, including longer periods of followup, to further confirm the efficacy of silicon matrix calcium phosphate as a safe and effective bone graft substitute.
Keywords: Spinal fusion graft; bone substitute; silicon matrix calcium phosphate.
Figures



Similar articles
-
The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: minimum 4-year follow-up of a pilot study.Spine J. 2008 May-Jun;8(3):457-65. doi: 10.1016/j.spinee.2007.03.012. Epub 2007 May 25. Spine J. 2008. PMID: 17588821 Clinical Trial.
-
Results of lumbar spondylodeses using different bone grafting materials after transforaminal lumbar interbody fusion (TLIF).Eur Spine J. 2017 Nov;26(11):2835-2842. doi: 10.1007/s00586-017-5145-0. Epub 2017 May 25. Eur Spine J. 2017. PMID: 28547573 Clinical Trial.
-
Comparison of a calcium phosphate bone substitute with recombinant human bone morphogenetic protein-2: a prospective study of fusion rates, clinical outcomes and complications with 24-month follow-up.Eur Spine J. 2017 Mar;26(3):754-763. doi: 10.1007/s00586-016-4927-0. Epub 2016 Dec 27. Eur Spine J. 2017. PMID: 28028645
-
Clinical outcomes and fusion rates following anterior lumbar interbody fusion with bone graft substitute i-FACTOR, an anorganic bone matrix/P-15 composite.J Neurosurg Spine. 2014 Dec;21(6):867-76. doi: 10.3171/2014.9.SPINE131151. Epub 2014 Oct 17. J Neurosurg Spine. 2014. PMID: 25325176
-
Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion.J Neurosurg Spine. 2014 Jul;21(1):106-32. doi: 10.3171/2014.4.SPINE14325. J Neurosurg Spine. 2014. PMID: 24980593 Review.
Cited by
-
Bone graft substitutes for anterior lumbar interbody fusion.Orthop Surg. 2013 May;5(2):77-85. doi: 10.1111/os.12030. Orthop Surg. 2013. PMID: 23658041 Free PMC article. Review.
-
Pain and disability after first-time spinal fusion for lumbar degenerative disorders: a systematic review and meta-analysis.Eur Spine J. 2019 Apr;28(4):696-709. doi: 10.1007/s00586-018-5680-3. Epub 2018 Jul 11. Eur Spine J. 2019. PMID: 29995169
References
-
- Becker S, Maissen O, Ponomarev I, Stoll T, Rahn B, Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine. 2006;31(1):11–17. - PubMed
-
- McConnell JR, Freeman BJ, Debnath UK, Grevitt MP, Prince HG, Webb JK. A prospective randomized comparison of coralline hydroxyapatite with autograft in cervical interbody fusion. Spine. 2003;28(4):317–323. - PubMed
-
- Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine. 2000;25(5):563–569. - PubMed
-
- Whang P, Wang J. Bone graft substitutes for spinal fusion. Spine J. 2003;3(2):155–156. - PubMed
-
- Hing KA, Merry JC, Gibson IR, Di-Silvio L, Best SM, Bonfield W. Bioceramics. In: Ohgushi H, Hastings GW, Yoshikawa T, editors. Vol. 12. Singapore: World Scientific; 1999. pp. 195–198.
LinkOut - more resources
Full Text Sources
