Prospective, Randomized, Multicenter FDA IDE Study of CHARITÉ Artificial Disc versus Lumbar Fusion: Effect at 5-year Follow-up of Prior Surgery and Prior Discectomy on Clinical Outcomes Following Lumbar Arthroplasty
- PMID: 25802625
- PMCID: PMC4365588
- DOI: 10.1016/SASJ-2008-0019-RR
Prospective, Randomized, Multicenter FDA IDE Study of CHARITÉ Artificial Disc versus Lumbar Fusion: Effect at 5-year Follow-up of Prior Surgery and Prior Discectomy on Clinical Outcomes Following Lumbar Arthroplasty
Abstract
Background: Candidates for spinal arthrodesis or arthroplasty often present with a history of prior surgery such as laminectomy, laminotomy or discectomy. In this study, lumbar arthroplasty patients with prior surgery, and in particular patients with prior discectomy, were evaluated for their clinical outcomes at the 5-year time point.
Methods: Randomized patients from the 5-year CHARITÉ investigational device exemption (IDE) study were divided as follows: 1) fusion prior surgery (excluding prior decompression with fusion) group (FSG); 2) fusion prior discectomy group (FDG); 3) fusion no prior surgery group (FNG); 4) arthroplasty prior surgery group (ASG); 5) arthroplasty prior discectomy group (ADG); and 6) arthroplasty no prior surgery group (ANG). The 5-year clinical outcomes included visual analog scale (VAS), Oswestry Disability Index 2.0 (ODI), patient satisfaction, and work status.
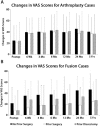
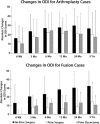
Results: In the arthroplasty group, all subgroups had statistically significant VAS improvements from baseline (VAS change from baseline: ASG = -36.6 ± 29.6, P < 0.0001; ADG = -40.2 ± 30.9, P = 0.0002; ANG = -36.5 ± 34.6, P < 0.0001). There was no statistical difference between subgroups (P = 0.5587). In the fusion group, VAS changes from baseline were statistically significant for the FNG and FSG subgroups, but not for the FDG patients (FNG = -46.3 ± 28.8, P < 0.0001; FSG = -24.2 ± 36.4, P = 0.0444; FDG = -26.7 ± 38.7, P = 0.2188). A trend of decreased VAS improvements was observed for FSG versus FNG (P = 0.0703) subgroups. Similar findings and trends were observed in ODI scores (Changes in ODI from baseline: ASG = -20.4 ± 23.8, P < 0.0001; ANG = -26.6±21.1, P < 0.0001; ADG= -17.6 ± 28.6, P = 0.0116; FSG = -14.5 ± 21.2, P = 0.0303; FNG= -32.5 ± 22.6, P < 0.0001; FDG = -10.7 ± 9.4, P = 0.0938). The greatest improvement in work status from preoperative to postoperative was seen in the ADG subgroup (28% increase in part- and full-time employment), while the FDG subgroup showed the greatest reduction in work status (17% decrease).
Conclusions: Arthroplasty patients with prior surgery or prior discectomy had similar clinical outcomes as arthroplasty patients without prior surgery, while fusion patients with prior surgery or prior discectomy showed trends of lowered clinical outcomes compared to fusion patients without prior surgery or discectomy.
Keywords: 5-year follow-up; arthrodesis; arthroplasty; clinical trial; lumbar; prior surgery.
Figures


References
-
- Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITÉ Artificial Disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565–1575. - PubMed
-
- Le Huec JC, Mathews H, Basso Y, et al. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36:315–322. - PubMed
-
- McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITÉ Artificial Disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:1576–1583. - PubMed
-
- Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155–1162. - PubMed
-
- Geisler FH, Guyer RD, Blumenthal SL, et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine. 2008;8:108–114. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
