Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface
- PMID: 25802873
- PMCID: PMC4329865
- DOI: 10.1155/2015/943056
Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface
Abstract
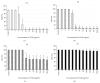
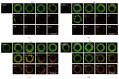
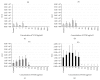
We investigated biofilm formation and time of vancomycin (VCM) resistance expression after adhesion to a metal surface in Staphylococcus epidermidis. Biofilm-forming Staphylococcus epidermidis with a VCM MIC of 1 μg/mL was used. The bacteria were made to adhere to a stainless steel washer and treated with VCM at different times and concentrations. VCM was administered 0, 2, 4, and 8 hours after adhesion. The amount of biofilm formed was evaluated based on the biofilm coverage rates (BCRs) before and after VCM administration, bacterial viability in biofilm was visually observed using the fluorescence staining method, and the viable bacterial count in biofilm was measured. The VCM concentration required to decrease BCR significantly compared with that of VCM-untreated bacteria was 4 μg/mL, even in the 0 hr group. In the 4 and 8 hr groups, VCM could not inhibit biofilm growth even at 1,024 μg/mL. In the 8 hr group, viable bacteria remained in biofilm at a count of 10(4) CFU even at a high VCM concentration (1,024 μg/mL). It was suggested that biofilm-forming Staphylococcus epidermidis expresses resistance to VCM early after adhesion to a metal surface. Resistance increased over time after adhesion as the biofilm formed, and strong resistance was expressed 4-8 hours after adhesion.
Figures

References
-
- Gristina A. G., Costerton J. W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. The Journal of Bone and Joint Surgery: American Volume. 1985;67(2):264–273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

