RNA Export through the NPC in Eukaryotes
- PMID: 25802992
- PMCID: PMC4377836
- DOI: 10.3390/genes6010124
RNA Export through the NPC in Eukaryotes
Abstract
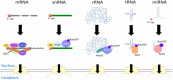
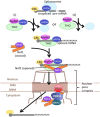
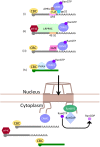
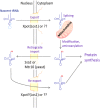
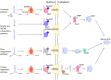
In eukaryotic cells, RNAs are transcribed in the nucleus and exported to the cytoplasm through the nuclear pore complex. The RNA molecules that are exported from the nucleus into the cytoplasm include messenger RNAs (mRNAs), ribosomal RNAs (rRNAs), transfer RNAs (tRNAs), small nuclear RNAs (snRNAs), micro RNAs (miRNAs), and viral mRNAs. Each RNA is transported by a specific nuclear export receptor. It is believed that most of the mRNAs are exported by Nxf1 (Mex67 in yeast), whereas rRNAs, snRNAs, and a certain subset of mRNAs are exported in a Crm1/Xpo1-dependent manner. tRNAs and miRNAs are exported by Xpot and Xpo5. However, multiple export receptors are involved in the export of some RNAs, such as 60S ribosomal subunit. In addition to these export receptors, some adapter proteins are required to export RNAs. The RNA export system of eukaryotic cells is also used by several types of RNA virus that depend on the machineries of the host cell in the nucleus for replication of their genome, therefore this review describes the RNA export system of two representative viruses. We also discuss the NPC anchoring-dependent mRNA export factors that directly recruit specific genes to the NPC.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

