Characterization of stimulus-secretion coupling in the human pancreatic EndoC-βH1 beta cell line
- PMID: 25803449
- PMCID: PMC4372368
- DOI: 10.1371/journal.pone.0120879
Characterization of stimulus-secretion coupling in the human pancreatic EndoC-βH1 beta cell line
Abstract
Aims/hypothesis: Studies on beta cell metabolism are often conducted in rodent beta cell lines due to the lack of stable human beta cell lines. Recently, a human cell line, EndoC-βH1, was generated. Here we investigate stimulus-secretion coupling in this cell line, and compare it with that in the rat beta cell line, INS-1 832/13, and human islets.
Methods: Cells were exposed to glucose and pyruvate. Insulin secretion and content (radioimmunoassay), gene expression (Gene Chip array), metabolite levels (GC/MS), respiration (Seahorse XF24 Extracellular Flux Analyzer), glucose utilization (radiometric), lactate release (enzymatic colorimetric), ATP levels (enzymatic bioluminescence) and plasma membrane potential and cytoplasmic Ca2+ responses (microfluorometry) were measured. Metabolite levels, respiration and insulin secretion were examined in human islets.
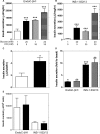
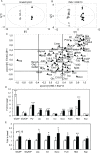
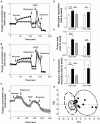
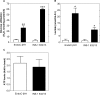
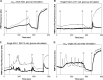
Results: Glucose increased insulin release, glucose utilization, raised ATP production and respiratory rates in both lines, and pyruvate increased insulin secretion and respiration. EndoC-βH1 cells exhibited higher insulin secretion, while plasma membrane depolarization was attenuated, and neither glucose nor pyruvate induced oscillations in intracellular calcium concentration or plasma membrane potential. Metabolite profiling revealed that glycolytic and TCA-cycle intermediate levels increased in response to glucose in both cell lines, but responses were weaker in EndoC-βH1 cells, similar to those observed in human islets. Respiration in EndoC-βH1 cells was more similar to that in human islets than in INS-1 832/13 cells.
Conclusions/interpretation: Functions associated with early stimulus-secretion coupling, with the exception of plasma membrane potential and Ca2+ oscillations, were similar in the two cell lines; insulin secretion, respiration and metabolite responses were similar in EndoC-βH1 cells and human islets. While both cell lines are suitable in vitro models, with the caveat of replicating key findings in isolated islets, EndoC-βH1 cells have the advantage of carrying the human genome, allowing studies of human genetic variants, epigenetics and regulatory RNA molecules.
Conflict of interest statement
Figures
References
-
- International_Diabetes_Federation (2013) IDF Diabetes Atlas. 6th ed. Brussels.
-
- Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414: 807–812. - PubMed
-
- Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes. 2004;53 Suppl 3: S113–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

