Complete protection of mice against lethal murine cytomegalovirus challenge by immunization with DNA vaccines encoding envelope glycoprotein complex III antigens gH, gL and gO
- PMID: 25803721
- PMCID: PMC4372543
- DOI: 10.1371/journal.pone.0119964
Complete protection of mice against lethal murine cytomegalovirus challenge by immunization with DNA vaccines encoding envelope glycoprotein complex III antigens gH, gL and gO
Abstract
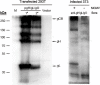
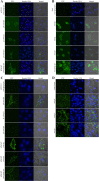
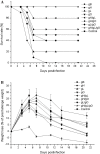
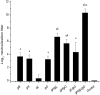
Human cytomegalovirus infects the majority of humanity which may lead to severe morbidity and mortality in newborns and immunocompromised adults. Humoral and cellular immunity are critical for controlling CMV infection. HCMV envelope glycoprotein complexes (gC I, II, III) represent major antigenic targets of antiviral immune responses. The gCIII complex is comprised of three glycoproteins, gH, gL, and gO. In the present study, DNA vaccines expressing the murine cytomegalovirus homologs of the gH, gL, and gO proteins were evaluated for protection against lethal MCMV infection in the mouse model. The results demonstrated that gH, gL, or gO single gene immunization could not yet offer good protection, whereas co-vaccination strategy apparently showed effects superior to separate immunization. Twice immunization with gH/gL/gO pDNAs could provide mice complete protection against lethal salivary gland-derived MCMV (SG-MCMV) challenge, while thrice immunization with pgH/pgL, pgH/pgO or pgL/pgO could not provide full protection. Co-vaccination with gH, gL and gO pDNAs elicited robust neutralizing antibody and cellular immune responses. Moreover, full protection was also achieved by simply passive immunization with anti-gH/gL/gO sera. These data demonstrated that gCIII complex antigens had fine immunogenicity and might be a promising candidate for the development of HCMV vaccines.
Conflict of interest statement
Figures
Similar articles
-
Immunization with cytomegalovirus envelope glycoprotein M and glycoprotein N DNA vaccines can provide mice with complete protection against a lethal murine cytomegalovirus challenge.Virol Sin. 2013 Jun;28(3):174-82. doi: 10.1007/s12250-013-3330-9. Epub 2013 May 24. Virol Sin. 2013. PMID: 23715998 Free PMC article.
-
Vectored co-delivery of human cytomegalovirus gH and gL proteins elicits potent complement-independent neutralizing antibodies.Vaccine. 2013 Jan 30;31(6):919-26. doi: 10.1016/j.vaccine.2012.12.009. Epub 2012 Dec 14. Vaccine. 2013. PMID: 23246547
-
Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication.J Virol. 2002 May;76(10):4822-35. doi: 10.1128/jvi.76.10.4822-4835.2002. J Virol. 2002. PMID: 11967299 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Humoral and cellular immune responses to herpes simplex virus-2 glycoprotein D generated by facilitated DNA immunization of mice.Curr Top Microbiol Immunol. 1998;226:79-89. doi: 10.1007/978-3-642-80475-5_6. Curr Top Microbiol Immunol. 1998. PMID: 9479837 Review. No abstract available.
Cited by
-
Importance of Highly Conserved Peptide Sites of Human Cytomegalovirus gO for Formation of the gH/gL/gO Complex.J Virol. 2016 Dec 16;91(1):e01339-16. doi: 10.1128/JVI.01339-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795411 Free PMC article.
-
Oral Vaccination with Attenuated Salmonella Expressing Viral M25 Protein Effectively Protects Mice Against Murine Cytomegalovirus Infection.Pathogens. 2025 Mar 25;14(4):314. doi: 10.3390/pathogens14040314. Pathogens. 2025. PMID: 40333046 Free PMC article.
-
Antiviral Immune Responses Against Murine Cytomegalovirus Induced by an Oral Salmonella-Based Vaccine Expressing Viral M33 Protein.Microorganisms. 2025 Jun 28;13(7):1510. doi: 10.3390/microorganisms13071510. Microorganisms. 2025. PMID: 40732019 Free PMC article.
-
Modeling Human Cytomegalovirus in Humanized Mice for Vaccine Testing.Vaccines (Basel). 2020 Feb 17;8(1):89. doi: 10.3390/vaccines8010089. Vaccines (Basel). 2020. PMID: 32079250 Free PMC article. Review.
-
Multi-Antigen Elephant Endotheliotropic Herpesvirus (EEHV) mRNA Vaccine Induces Humoral and Cell-Mediated Responses in Mice.Vaccines (Basel). 2024 Dec 18;12(12):1429. doi: 10.3390/vaccines12121429. Vaccines (Basel). 2024. PMID: 39772089 Free PMC article.
References
-
- Morello CS, Kelley LA, Munks MW, Hill AB, Spector DH. DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. J Virol. 2007; 81: 7766–7775. - PMC - PubMed
-
- Zhong J, Khanna R. Vaccine strategies against human cytomegalovirus infection. Expert Review of Anti-Infective Therapy. 2007; 5: 449–459. - PubMed
-
- Griffiths PD. The 2001 Garrod Lecture—The treatment of cytomegalovirus infection. Journal of Antimicrobial Chemotherapy. 2002; 49: 243–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

