Screening of drugs to treat 8p11 myeloproliferative syndrome using patient-derived induced pluripotent stem cells with fusion gene CEP110-FGFR1
- PMID: 25803811
- PMCID: PMC4372437
- DOI: 10.1371/journal.pone.0120841
Screening of drugs to treat 8p11 myeloproliferative syndrome using patient-derived induced pluripotent stem cells with fusion gene CEP110-FGFR1
Abstract
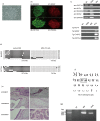
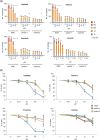
Induced pluripotent stem (iPS) cells provide powerful tools for studying disease mechanisms and developing therapies for diseases. The 8p11 myeloproliferative syndrome (EMS) is an aggressive chronic myeloproliferative disorder (MPD) that is caused by constitutive activation of fibroblast growth factor receptor 1. EMS is rare and, consequently, effective treatment for this disease has not been established. Here, iPS cells were generated from an EMS patient (EMS-iPS cells) to assist the development of effective therapies for EMS. When iPS cells were co-cultured with murine embryonic stromal cells, EMS-iPS cells produced more hematopoietic progenitor and hematopoietic cells, and CD34+ cells derived from EMS-iPS cells exhibited 3.2-7.2-fold more macrophage and erythroid colony forming units (CFUs) than those derived from control iPS cells. These data indicate that EMS-iPS cells have an increased hematopoietic differentiation capacity, which is characteristic of MPDs. To determine whether a tyrosine kinase inhibitor (TKI) could suppress the increased number of CFUs formed by EMS-iPS-induced CD34+ cells, cells were treated with one of four TKIs (CHIR258, PKC 412, ponatinib, and imatinib). CHIR258, PKC 412, and ponatinib reduced the number of CFUs formed by EMS-iPS-induced CD34+ cells in a dose-dependent manner, whereas imatinib did not. Similar effects were observed on primary peripheral blood cells (more than 90% of which were blasts) isolated from the patient. This study provides evidence that the EMS-iPS cell line is a useful tool for the screening of drugs to treat EMS and to investigate the mechanism underlying this disease.
Conflict of interest statement
Figures


References
-
- Macdonald D, Aguiar RC, Mason PJ, Goldman JM, Cross NC. A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia. 1995;9(10):1628–30. Epub 1995/10/01. PubMed . - PubMed
-
- Macdonald D, Reiter A, Cross NC. The 8p11 myeloproliferative syndrome: a distinct clinical entity caused by constitutive activation of FGFR1. Acta haematologica. 2002;107(2):101–7. Epub 2002/03/29. doi: 46639. PubMed . - PubMed
-
- Guasch G, Mack GJ, Popovici C, Dastugue N, Birnbaum D, Rattner JB, et al. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33). Blood. 2000;95(5):1788–96. Epub 2000/02/26. PubMed . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

