Avoidable waste of research related to inadequate methods in clinical trials
- PMID: 25804210
- PMCID: PMC4372296
- DOI: 10.1136/bmj.h809
Avoidable waste of research related to inadequate methods in clinical trials
Abstract
Objective: To assess the waste of research related to inadequate methods in trials included in Cochrane reviews and to examine to what extent this waste could be avoided. A secondary objective was to perform a simulation study to re-estimate this avoidable waste if all trials were adequately reported.
Design: Methodological review and simulation study.
Data sources: Trials included in the meta-analysis of the primary outcome of Cochrane reviews published between April 2012 and March 2013.
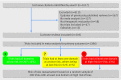
Data extraction and synthesis: We collected the risk of bias assessment made by the review authors for each trial. For a random sample of 200 trials with at least one domain at high risk of bias, we re-assessed risk of bias and identified all related methodological problems. For each problem, possible adjustments were proposed that were then validated by an expert panel also evaluating their feasibility (easy or not) and cost. Avoidable waste was defined as trials with at least one domain at high risk of bias for which easy adjustments with no or minor cost could change all domains to low risk. In the simulation study, after extrapolating our re-assessment of risk of bias to all trials, we considered each domain rated as unclear risk of bias as missing data and used multiple imputations to determine whether they were at high or low risk.
Results: Of 1286 trials from 205 meta-analyses, 556 (43%) had at least one domain at high risk of bias. Among the sample of 200 of these trials, 142 were confirmed as high risk; in these, we identified 25 types of methodological problem. Adjustments were possible in 136 trials (96%). Easy adjustments with no or minor cost could be applied in 71 trials (50%), resulting in 17 trials (12%) changing to low risk for all domains. So the avoidable waste represented 12% (95% CI 7% to 18%) of trials with at least one domain at high risk. After correcting for incomplete reporting, avoidable waste due to inadequate methods was estimated at 42% (95% CI 36% to 49%).
Conclusions: An important burden of wasted research is related to inadequate methods. This waste could be partly avoided by simple and inexpensive adjustments.
© Yordanov et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
[Methodology problems: avoiding "garbage" in clinical research].Dtsch Med Wochenschr. 2015 May;140(10):710. Dtsch Med Wochenschr. 2015. PMID: 26171474 German. No abstract available.
References
-
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9. - PubMed
-
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65. - PubMed
-
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
