Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy
- PMID: 25804280
- PMCID: PMC4587688
- DOI: 10.1681/ASN.2014070640
Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy
Abstract
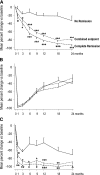
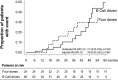
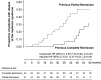
Rituximab induces nephrotic syndrome (NS) remission in two-thirds of patients with primary membranous nephropathy (MN), even after other treatments have failed. To assess the relationships among treatment effect, circulating nephritogenic anti-phospholipase A2 receptor (anti-PLA2R) autoantibodies and genetic polymorphisms predisposing to antibody production we serially monitored 24-hour proteinuria and antibody titer in patients with primary MN and long-lasting NS consenting to rituximab (375 mg/m(2)) therapy and genetic analyses. Over a median (range) follow-up of 30.8 (6.0-145.4) months, 84 of 132 rituximab-treated patients achieved complete or partial NS remission (primary end point), and 25 relapsed after remission. Outcomes of patients with or without detectable anti-PLA2R antibodies at baseline were similar. Among the 81 patients with antibodies, lower anti-PLA2R antibody titer at baseline (P=0.001) and full antibody depletion 6 months post-rituximab (hazard ratio [HR], 7.90; 95% confidence interval [95% CI], 2.54 to 24.60; P<0.001) strongly predicted remission. All 25 complete remissions were preceded by complete anti-PLA2R antibody depletion. On average, 50% anti-PLA2R titer reduction preceded equivalent proteinuria reduction by 10 months. Re-emergence of circulating antibodies predicted disease relapse (HR, 6.54; 95% CI, 1.57 to 27.40; P=0.01), whereas initial complete remission protected from the event (HR, 6.63; 95% CI, 2.37 to 18.53; P<0.001). Eighteen patients achieved persistent antibody depletion and complete remission and never relapsed. Outcome was independent of PLA2R1 and HLA-DQA1 polymorphisms and of previous immunosuppressive treatment. Therefore, assessing circulating anti-PLA2R autoantibodies and proteinuria may help in monitoring disease activity and guiding personalized rituximab therapy in nephrotic patients with primary MN.
Keywords: glomerulonephritis; membranous nephropathy; nephrotic syndrome; polymorphisms; primary; proteinuria.
Copyright © 2015 by the American Society of Nephrology.
Figures






Comment in
-
Anti-phospholipase A2 Receptor Antibody and Immunosuppression in Membranous Nephropathy: More Evidence for Pathogenicity of Anti-phospholipase A2 Receptor Autoantibodies.J Am Soc Nephrol. 2015 Oct;26(10):2308-11. doi: 10.1681/ASN.2015020181. Epub 2015 Mar 24. J Am Soc Nephrol. 2015. PMID: 25804283 Free PMC article. No abstract available.
References
-
- Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 - PubMed
-
- Cravedi P, Sghirlanzoni MC, Marasà M, Salerno A, Remuzzi G, Ruggenenti P: Efficacy and safety of rituximab second-line therapy for membranous nephropathy: A prospective, matched-cohort study. Am J Nephrol 33: 461–468, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

