Arabidopsis RIC1 Severs Actin Filaments at the Apex to Regulate Pollen Tube Growth
- PMID: 25804540
- PMCID: PMC4558691
- DOI: 10.1105/tpc.114.135400
Arabidopsis RIC1 Severs Actin Filaments at the Apex to Regulate Pollen Tube Growth
Abstract
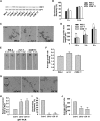
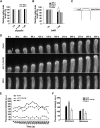
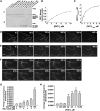
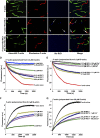
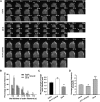
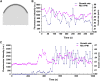
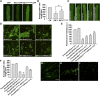
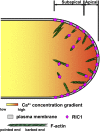
Pollen tubes deliver sperms to the ovule for fertilization via tip growth. The rapid turnover of F-actin in pollen tube tips plays an important role in this process. In this study, we demonstrate that Arabidopsis thaliana RIC1, a member of the ROP-interactive CRIB motif-containing protein family, regulates pollen tube growth via its F-actin severing activity. Knockout of RIC1 enhanced pollen tube elongation, while overexpression of RIC1 dramatically reduced tube growth. Pharmacological analysis indicated that RIC1 affected F-actin dynamics in pollen tubes. In vitro biochemical assays revealed that RIC1 directly bound and severed F-actin in the presence of Ca(2+) in addition to interfering with F-actin turnover by capping F-actin at the barbed ends. In vivo, RIC1 localized primarily to the apical plasma membrane (PM) of pollen tubes. The level of RIC1 at the apical PM oscillated during pollen tube growth. The frequency of F-actin severing at the apex was notably decreased in ric1-1 pollen tubes but was increased in pollen tubes overexpressing RIC1. We propose that RIC1 regulates F-actin dynamics at the apical PM as well as the cytosol by severing F-actin and capping the barbed ends in the cytoplasm, establishing a novel mechanism that underlies the regulation of pollen tube growth.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures


Similar articles
-
MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization.Plant Cell. 2013 Mar;25(3):851-67. doi: 10.1105/tpc.113.110528. Epub 2013 Mar 5. Plant Cell. 2013. PMID: 23463774 Free PMC article.
-
Arabidopsis villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars.Plant Cell. 2013 May;25(5):1803-17. doi: 10.1105/tpc.113.110940. Epub 2013 May 28. Plant Cell. 2013. PMID: 23715472 Free PMC article.
-
Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments.Plant Cell. 2014 Jan;26(1):325-39. doi: 10.1105/tpc.113.119768. Epub 2014 Jan 14. Plant Cell. 2014. PMID: 24424096 Free PMC article.
-
ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin.J Exp Bot. 2003 Jan;54(380):93-101. doi: 10.1093/jxb/erg035. J Exp Bot. 2003. PMID: 12456759 Review.
-
Regulation of actin dynamics in pollen tubes: control of actin polymer level.J Integr Plant Biol. 2009 Aug;51(8):740-50. doi: 10.1111/j.1744-7909.2009.00850.x. J Integr Plant Biol. 2009. PMID: 19686371 Review.
Cited by
-
The quest for the central players governing pollen tube growth and guidance.Plant Physiol. 2021 Apr 2;185(3):682-693. doi: 10.1093/plphys/kiaa092. Plant Physiol. 2021. PMID: 33793904 Free PMC article. Review.
-
Hydrogen Sulfide Disturbs Actin Polymerization via S-Sulfhydration Resulting in Stunted Root Hair Growth.Plant Physiol. 2018 Oct;178(2):936-949. doi: 10.1104/pp.18.00838. Epub 2018 Aug 30. Plant Physiol. 2018. PMID: 30166418 Free PMC article.
-
Polarized subcellular activation of Rho proteins by specific ROPGEFs drives pollen germination in Arabidopsis thaliana.PLoS Biol. 2025 Apr 21;23(4):e3003139. doi: 10.1371/journal.pbio.3003139. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40258071 Free PMC article.
-
Establishing cell polarity in plants: the role of cytoskeletal structures and regulatory pathways.Front Cell Dev Biol. 2025 May 9;13:1602463. doi: 10.3389/fcell.2025.1602463. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40417180 Free PMC article. Review.
-
Chloroplast avoidance movement: a novel paradigm of ROS signalling.Photosynth Res. 2020 Apr;144(1):109-121. doi: 10.1007/s11120-020-00736-9. Epub 2020 Mar 28. Photosynth Res. 2020. PMID: 32222888 Review.
References
-
- Boavida L.C., McCormick S. (2007). Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 52: 570–582. - PubMed
-
- Bou Daher F., Geitmann A. (2011). Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic 12: 1537–1551. - PubMed
-
- Cheung A.Y., Wu H.M. (2008). Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu. Rev. Plant Biol. 59: 547–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

