Effects of metformin, buformin, and phenformin on the post-initiation stage of chemically induced mammary carcinogenesis in the rat
- PMID: 25804611
- PMCID: PMC4452421
- DOI: 10.1158/1940-6207.CAPR-14-0121
Effects of metformin, buformin, and phenformin on the post-initiation stage of chemically induced mammary carcinogenesis in the rat
Abstract
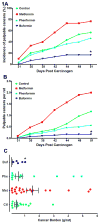
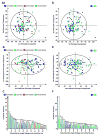
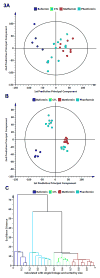
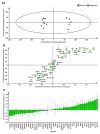
Metformin is a widely prescribed drug for the treatment of type II diabetes. Although epidemiologic data have provided a strong rationale for investigating the potential of this biguanide for use in cancer prevention and control, uncertainty exists whether metformin should be expected to have an impact in nondiabetic patients. Furthermore, little attention has been given to the possibility that other biguanides may have anticancer activity. In this study, the effects of clinically relevant doses of metformin (9.3 mmol/kg diet), buformin (7.6 mmol/kg diet), and phenformin (5.0 mmol/kg diet) were compared with rats fed control diet (AIN93-G) during the post-initiation stage of 1-methyl-1-nitrosourea-induced (50 mg/kg body weight) mammary carcinogenesis (n = 30/group). Plasma, liver, skeletal muscle, visceral fat, mammary gland, and mammary carcinoma concentrations of the biguanides were determined. In comparison with the control group, buformin decreased cancer incidence, multiplicity, and burden, whereas metformin and phenformin had no statistically significant effect on the carcinogenic process relative to the control group. Buformin did not alter fasting plasma glucose or insulin. Within mammary carcinomas, evidence was obtained that buformin treatment perturbed signaling pathways related to energy sensing. However, further investigation is needed to determine the relative contributions of host systemic and cell autonomous mechanisms to the anticancer activity of biguanides such as buformin.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Azvolinsky A. Repurposing to fight cancer: the metformin-prostate cancer connection. J Natl Cancer Inst. 2014;106:dju030. - PubMed
-
- Yue W, Yang CS, Dipaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila) 2014 - PubMed
-
- Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–80. - PubMed
-
- Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. - PubMed
-
- Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

