WT1 targets Gas1 to maintain nephron progenitor cells by modulating FGF signals
- PMID: 25804736
- PMCID: PMC4378252
- DOI: 10.1242/dev.119735
WT1 targets Gas1 to maintain nephron progenitor cells by modulating FGF signals
Abstract
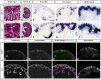
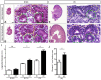
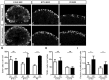
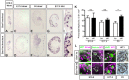
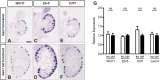
Development of the metanephric kidney depends on tightly regulated interplay between self-renewal and differentiation of a nephron progenitor cell (NPC) pool. Several key factors required for the survival of NPCs have been identified, including fibroblast growth factor (FGF) signaling and the transcription factor Wilms' tumor suppressor 1 (WT1). Here, we present evidence that WT1 modulates FGF signaling by activating the expression of growth arrest-specific 1 (Gas1), a novel WT1 target gene and novel modulator of FGF signaling. We show that WT1 directly binds to a conserved DNA binding motif within the Gas1 promoter and activates Gas1 mRNA transcription in NPCs. We confirm that WT1 is required for Gas1 expression in kidneys in vivo. Loss of function of GAS1 in vivo results in hypoplastic kidneys with reduced nephron mass due to premature depletion of NPCs. Although kidney development in Gas1 knockout mice progresses normally until E15.5, NPCs show decreased rates of proliferation at this stage and are depleted as of E17.5. Lastly, we show that Gas1 is selectively required for FGF-stimulated AKT signaling in vitro. In summary, our data suggest a model in which WT1 modulates receptor tyrosine kinase signaling in NPCs by directing the expression of Gas1.
Keywords: Fibroblast growth factor signaling; Kidney development; Mouse; Nephron progenitor cell.
© 2015. Published by The Company of Biologists Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

