Activin A directs striatal projection neuron differentiation of human pluripotent stem cells
- PMID: 25804741
- PMCID: PMC4378247
- DOI: 10.1242/dev.117093
Activin A directs striatal projection neuron differentiation of human pluripotent stem cells
Abstract
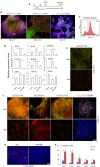
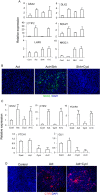
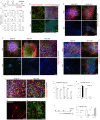
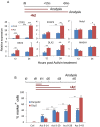
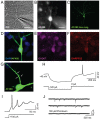
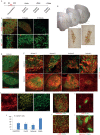
The efficient generation of striatal neurons from human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) is fundamental for realising their promise in disease modelling, pharmaceutical drug screening and cell therapy for Huntington's disease. GABAergic medium-sized spiny neurons (MSNs) are the principal projection neurons of the striatum and specifically degenerate in the early phase of Huntington's disease. Here we report that activin A induces lateral ganglionic eminence (LGE) characteristics in nascent neural progenitors derived from hESCs and hiPSCs in a sonic hedgehog-independent manner. Correct specification of striatal phenotype was further demonstrated by the induction of the striatal transcription factors CTIP2, GSX2 and FOXP2. Crucially, these human LGE progenitors readily differentiate into postmitotic neurons expressing the striatal projection neuron signature marker DARPP32, both in culture and following transplantation in the adult striatum in a rat model of Huntington's disease. Activin-induced neurons also exhibit appropriate striatal-like electrophysiology in vitro. Together, our findings demonstrate a novel route for efficient differentiation of GABAergic striatal MSNs from human pluripotent stem cells.
Keywords: Activin; DARPP32 (PPP1R1B); Huntington's disease; Lateral ganglionic eminence; Medium spiny neuron; Neural differentiation; Pluripotent stem cell; Striatum; Transplantation.
© 2015. Published by The Company of Biologists Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

