Long-term follow-up of rituximab plus first-line mitoxantrone, chlorambucil, prednisolone and interferon-alpha as maintenance therapy in follicular lymphoma
- PMID: 25804839
- PMCID: PMC11823963
- DOI: 10.1007/s00432-015-1963-9
Long-term follow-up of rituximab plus first-line mitoxantrone, chlorambucil, prednisolone and interferon-alpha as maintenance therapy in follicular lymphoma
Abstract
Purpose: The randomised, controlled OSHO#39 study showed promising results using first-line mitoxantrone, chlorambucil and prednisolone (MCP) chemotherapy plus rituximab in patients with advanced symptomatic follicular lymphoma (FL) in need of therapy. The aim of this long-term follow-up was to investigate whether clinical benefits are maintained after up to 9 years of observation.
Methods: Following the 4-year follow-up of OSHO#39, 77 FL patients who received rituximab plus MCP (R-MCP) and 52 patients who received MCP (129 patients alive and not previously censored in total) were followed for 5 additional years in this prospective, non-interventional, observational study. For the efficacy analysis, data were jointly analysed with OSHO#39 data (FL intention-to-treat population: 105 patients R-MCP, 96 MCP). Patients not included in the 5-year follow-up were censored.
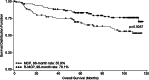
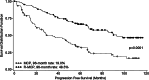
Results: For surviving patients, median follow-up was 102 months (R-MCP) and 87 months (MCP). Although median overall survival (OS) was not yet reached, OS was longer for patients with R-MCP compared with MCP (p = 0.0057), with 8-year-survival rates of 76.1 versus 55.9%. Further time-to-event data were substantially longer for the R-MCP group than for MCP alone: median progression-free survival (PFS) was 93.4 versus 34.9 months, and median event-free survival (EFS) 89.6 versus 26.5 months. Unplanned subanalyses of patients with and without interferon maintenance showed improved PFS and EFS without an impact on OS.
Conclusions: The addition of rituximab to first-line MCP chemotherapy improves clinical outcomes in advanced FL patients and translates into long-term OS benefits. R-MCP remains a promising standard option for this patient group.
Conflict of interest statement
Michael Herold: Honoraria, advisory board, research support from Roche Pharma AG. Christian Scholz: Roche speaker fees, advisory board, travel support. Frank Rothmann: No conflict of interest. Carsten Hirt: Travel and research funding from Roche Pharma AG. Volker Lakner: No conflict of interest. Ralph Naumann: No conflict of interest.
Figures
References
-
- Buske C et al (2008) Rituximab in combination with CHOP in patients with follicular lymphoma: analysis of treatment outcome of 552 patients treated in a randomized trial of the German Low Grade Lymphoma Study Group (GLSG) after a follow up of 58 months Blood (ASH Annual Meeting Abstracts) 112:Abstract 2599
-
- Buske C, Herold M, Willenbacher W, Dreyling M (2012) Follikuläres Lymphom (FL). DGHO-Onkopedia-Leitlinie. http://www.dgho-onkopedia.de/de/onkopedia/leitlinien/follikulaeres-lymph.... Zugriff am 2.9.2013
-
- Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Group EGW (2011) Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):vi59–vi63. doi:10.1093/annonc/mdr388 - PubMed
-
- Forstpointner R et al (2004) The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104:3064–3071. doi:10.1182/blood-2004-04-1323 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

