Botulinum Toxin-induced Muscle Paralysis Inhibits Heterotopic Bone Formation
- PMID: 25804882
- PMCID: PMC4523519
- DOI: 10.1007/s11999-015-4271-4
Botulinum Toxin-induced Muscle Paralysis Inhibits Heterotopic Bone Formation
Abstract
Background: Short-term muscle atrophy induced by botulinum toxin A (BTxA) has been observed to impair osteogenesis in a rat closed femur fracture model. However, it is unclear whether the underlying mechanism is a direct effect of BTxA on muscle-bone interactions or an indirect effect that is driven by skeletal unloading. Because skeletal trauma in the closed fracture model also leads to disuse atrophy, we sought to mitigate this confounding variable by examining BTxA effects on muscle-bone interactions in two complementary in vivo models in which osteogenesis is induced in the absence of skeletal unloading. The overall aim of this study was to identify a potential strategy to inhibit pathological bone formation and heterotopic ossification (HO).
Questions/purposes: (1) Does muscle paralysis inhibit periosteal osteogenesis induced by a transcortical defect? (2) Does muscle paralysis inhibit heterotopic bone formation stimulated by intramuscular bone morphogenetic protein (BMP) injection?
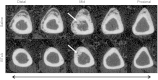
Methods: Focal osteogenesis was induced in the right hindlimb of mice through surgical initiation of a small transcortical defect in the tibia (fracture callus; n = 7/group) or intramuscular injection of BMP-2 (HO lesion; n = 6/group), both in the presence/absence of adjacent calf paralysis. High-resolution micro-CT images were obtained in all experimental groups 21 days postinduction and total volume (ie, perimeter of periosteal callus or HO lesion) and bone volume (calcified tissue within the total volume) were quantified as primary outcome measures. Finally, these outcome measures were compared to determine the effect of muscle paralysis on inhibition of local osteogenesis in both studies.
Results: After a transcortical defect, BTxA-treated mice showed profound inhibition of osteogenesis in the periosteal fracture callus 21 days postsurgery compared with saline-treated mice (total volume: 0.08 ± 0.06 versus 0.42 ± 0.11 mm(3), p < 0.001; bone volume: 0.07 ± 0.05 versus 0.32 ± 0.07 mm(3), p < 0.001). Similarly, BMP-2-induced HO formation was inhibited by adjacent muscle paralysis at the same time point (total volume: 1.42 ± 0.31 versus 3.42 ± 2.11 mm(3), p = 0.034; bone volume: 0.68 ± 0.18 versus 1.36 ± 0.79 mm(3), p = 0.045).
Conclusions: Our data indicate that BTxA-induced neuromuscular inhibition mitigated osteogenesis associated with both a transcortical defect and BMP-2-induced HO.
Clinical relevance: Focal neuromuscular inhibition represents a promising new approach that may lead to a new clinical intervention to mitigate trauma-induced HO, a healthcare challenge that is severely debilitating for civilian and war-wounded populations, is costly to both the patient and the healthcare system, and currently lacks effective treatments.
Figures




References
-
- Ausk BJ, Huber P, Srinivasan S, Bain SD, Kwon RY, McNamara EA, Poliachik SL, Sybrowsky CL, Gross TS. Metaphyseal and diaphyseal bone loss in the tibia following transient muscle paralysis are spatiotemporally distinct resorption events. Bone. 2013;57:413–422. doi: 10.1016/j.bone.2013.09.009. - DOI - PMC - PubMed
-
- Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

