Total synthesis of glycosylated proteins
- PMID: 25805144
- PMCID: PMC5079620
- DOI: 10.1007/128_2014_622
Total synthesis of glycosylated proteins
Abstract
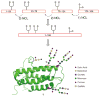
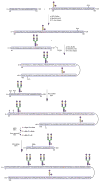
Glycoproteins are an important class of naturally occurring biomolecules which play a pivotal role in many biological processes. They are biosynthesized as complex mixtures of glycoforms through post-translational protein glycosylation. This fact, together with the challenges associated with producing them in homogeneous form, has hampered detailed structure-function studies of glycoproteins as well as their full exploitation as potential therapeutic agents. By contrast, chemical synthesis offers the unique opportunity to gain access to homogeneous glycoprotein samples for rigorous biological evaluation. Herein, we review recent methods for the assembly of complex glycopeptides and glycoproteins and present several examples from our laboratory towards the total chemical synthesis of clinically relevant glycosylated proteins that have enabled synthetic access to full-length homogeneous glycoproteins.
Figures











References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

