Type IV pilus secretins have extracellular C termini
- PMID: 25805731
- PMCID: PMC4453517
- DOI: 10.1128/mBio.00322-15
Type IV pilus secretins have extracellular C termini
Abstract
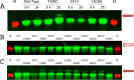
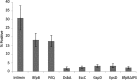
Type IV pili (T4Ps) are surface appendages used by Gram-negative and Gram-positive pathogens for motility and attachment to epithelial surfaces. In Gram-negative bacteria, such as the important pediatric pathogen enteropathogenic Escherichia coli (EPEC), during extension and retraction, the pilus passes through an outer membrane (OM) pore formed by the multimeric secretin complex. The secretin is common to Gram-negative assemblies, including the related type 2 secretion (T2S) system and the type 3 secretion (T3S) system. The N termini of the secretin monomers are periplasmic and in some systems have been shown to mediate substrate specificity. In this study, we mapped the topology of BfpB, the T4P secretin from EPEC, using a combination of biochemical and biophysical techniques that allowed selective identification of periplasmic and extracellular residues. We applied rules based on solved atomic structures of outer membrane proteins (OMPs) to generate our topology model, combining the experimental results with secondary structure prediction algorithms and direct inspection of the primary sequence. Surprisingly, the C terminus of BfpB is extracellular, a result confirmed by flow cytometry for BfpB and a distantly related T4P secretin, PilQ, from Pseudomonas aeruginosa. Keeping with prior evidence, the C termini of two T2S secretins and one T3S secretin were not detected on the extracellular surface. On the basis of our data and structural constraints, we propose that BfpB forms a beta barrel with 16 transmembrane beta strands. We propose that the T4P secretins have a C-terminal segment that passes through the center of each monomer.
Importance: Secretins are multimeric proteins that allow the passage of secreted toxins and surface structures through the outer membranes (OMs) of Gram-negative bacteria. To date, there have been no atomic structures of the C-terminal region of a secretin, although electron microscopy (EM) structures of the complex are available. This work provides a detailed topology prediction of the membrane-spanning domain of a type IV pilus (T4P) secretin. Our study used innovative techniques to provide new and comprehensive information on secretin topology, highlighting similarities and differences among secretin subfamilies. Additionally, the techniques used in this study may prove useful for the study of other OM proteins.
Copyright © 2015 Lieberman et al.
Figures



References
-
- Fröls S, Ajon M, Wagner M, Teichmann D, Zolghadr B, Folea M, Boekema EJ, Driessen AJ, Schleper C, Albers SV. 2008. UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation. Mol Microbiol 70:938–952. doi:10.1111/j.1365-2958.2008.06459.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

