The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial
- PMID: 25805886
- PMCID: PMC4466187
- DOI: 10.1093/schbul/sbv022
The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial
Abstract
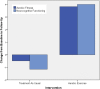
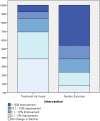
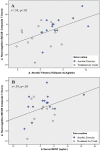
Individuals with schizophrenia display substantial neurocognitive deficits for which available treatments offer only limited benefits. Yet, findings from studies of animals, clinical and nonclinical populations have linked neurocognitive improvements to increases in aerobic fitness (AF) via aerobic exercise training (AE). Such improvements have been attributed to up-regulation of brain-derived neurotrophic factor (BDNF). However, the impact of AE on neurocognition, and the putative role of BDNF, have not been investigated in schizophrenia. Employing a proof-of-concept, single-blind, randomized clinical trial design, 33 individuals with schizophrenia were randomized to receive standard psychiatric treatment (n = 17; "treatment as usual"; TAU) or attend a 12-week AE program (n = 16) utilizing active-play video games (Xbox 360 Kinect) and traditional AE equipment. Participants completed assessments of AF (indexed by VO2 peak ml/kg/min), neurocognition (MATRICS Consensus Cognitive Battery), and serum-BDNF before and after and 12-week period. Twenty-six participants (79%) completed the study. At follow-up, the AE participants improved their AF by 18.0% vs a -0.5% decline in the TAU group (P = .002) and improved their neurocognition by 15.1% vs -2.0% decline in the TAU group (P = .031). Hierarchical multiple regression analyses indicated that enhancement in AF and increases in BDNF predicted 25.4% and 14.6% of the neurocognitive improvement variance, respectively. The results indicate AE is effective in enhancing neurocognitive functioning in people with schizophrenia and provide preliminary support for the impact of AE-related BDNF up-regulation on neurocognition in this population. Poor AF represents a modifiable risk factor for neurocognitive dysfunction in schizophrenia for which AE training offer a safe, nonstigmatizing, and side-effect-free intervention.
Keywords: aerobic fitness; brain-derived neurotrophic factor/active-play video games; cognition; neurotrophins.
© The Author 2015. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. - PubMed
-
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. - PubMed
-
- Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

