Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson's disease
- PMID: 25805964
- PMCID: PMC4353246
- DOI: 10.3389/fnins.2015.00059
Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson's disease
Abstract
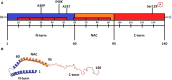
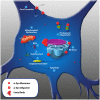
Alpha-synuclein is a presynaptic protein expressed throughout the central nervous system, and it is the main component of Lewy bodies, one of the histopathological features of Parkinson's disease (PD) which is a progressive and irreversible neurodegenerative disorder. The conformational flexibility of α-synuclein allows it to adopt different conformations, i.e., bound to membranes or form aggregates, the oligomers are believed to be the more toxic species. In this review, we will focus on two major features of α-synuclein, transmission and toxicity, that could help to understand the pathological characteristics of PD. One important feature of α-synuclein is its ability to be transmitted from neuron to neuron using mechanisms such as endocytosis, plasma membrane penetration or through exosomes, thus propagating the Lewy body pathology to different brain regions thereby contributing to the progressiveness of PD. The second feature of α-synuclein is that it confers cytotoxicity to recipient cells, principally when it is in an oligomeric state. This form causes mitochondrial dysfunction, endoplasmic reticulum stress, oxidative stress, proteasome impairment, disruption of plasma membrane and pore formation that lead to apoptosis pathway activation and consequent cell death. The complexity of α-synuclein oligomerization and formation of toxic species could be a major factor for the irreversibility of PD and could also explain the lack of successful therapies to halt the disease.
Keywords: Parkinson's disease; alpha-synuclein; neurodegeneration; toxicity; transmission.
Figures

References
-
- Aguayo L. G., Parodi J., Sepúlveda F. J., Opazo C. (2009). Pore-forming neurotoxin-like mechanism for Aβ oligomer-induced synaptic failure, in Pore-Forming Neurotoxin-Like Mechanism for Aβ Oligomer-Induced Synaptic Failure, eds Perry G., Maccioni R. B. (New York, NY: Springer; ), 13–21 10.1007/978-0-387-87995-6 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

