γδ T cell therapy for the treatment of non-small cell lung cancer
- PMID: 25806278
- PMCID: PMC4367606
- DOI: 10.3978/j.issn.2218-6751.2013.11.01
γδ T cell therapy for the treatment of non-small cell lung cancer
Abstract
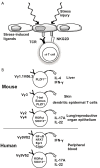
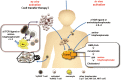
γδ T cells are attractive effector cells for cancer immunotherapy as they can secrete cytokines abundantly and exert potent cytotoxicity against a wide range of cancer cells. They comprise 1-5% of peripheral blood T cells, the majority expressing the Vγ9Vδ2 T cell receptor that recognizes phosphoantigens. Direct in vivo activation of Vγ9Vδ2 T cells in cancer patients as well as adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells has been investigated in several clinical trials. We previously established a large-scale in vitro expansion method for Vγ9Vδ2 T cells using zoledronate and interleukin-2 (IL-2). We found that Vγ9Vδ2 T cells from patients with advanced cancer as well as from healthy donors underwent extensive proliferation under these conditions. Such cultured Vγ9Vδ2 T cells retained cytokine secretion capacity and mediated cytotoxicity against a variety of cancer cell lines. Recently, we conducted a phase I clinical study to evaluate safety and potential anti-tumor effects of re-infusing ex vivo expanded γδ T cells in patients with advanced or recurrent non-small-cell lung cancer (NSCLC) refractory to or intolerant of current conventional treatments. There were no severe adverse events related to the therapy. All patients remained alive during the study period with a median survival of 589 days and median progression-free survival of 126 days. Six patients had stable disease (SD), whereas the remaining six evaluable patients experienced progressive disease (PD) four weeks after the sixth transfer. We conclude that adoptive transfer of zoledronate-expanded γδ T cells is safe and feasible in patients with NSCLC, refractory to other treatments.
Keywords: Non-small-cell lung cancer (NSCLC); adoptive transfer; cell therapy; γδ T cells.
Figures



References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. - PubMed
-
- Bonanno L, Favaretto A, Rugge M, et al. Role of genotyping in non-small cell lung cancer treatment: current status. Drugs 2011;71:2231-46. - PubMed
-
- Kakimi K, Nakajima J, Wada H.Active specific immunotherapy and cell-transfer therapy for the treatment of non-small cell lung cancer. Lung Cancer 2009;65:1-8. - PubMed
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000;18:975-1026. - PubMed
-
- Pont F, Familiades J, Déjean S, et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of αβ T-cell and NK-cell signatures. Eur J Immunol 2012;42:228-40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
