The Effect of Progestins on Tumor Necrosis Factor α-Induced Matrix Metalloproteinase-9 Activity and Gene Expression in Human Primary Amnion and Chorion Cells In Vitro
- PMID: 25806402
- PMCID: PMC4406818
- DOI: 10.1213/ANE.0000000000000708
The Effect of Progestins on Tumor Necrosis Factor α-Induced Matrix Metalloproteinase-9 Activity and Gene Expression in Human Primary Amnion and Chorion Cells In Vitro
Abstract
Background: Current treatment modalities for preventing preterm premature rupture of membranes are limited, but progestins may play a role. Tumor necrosis factor α (TNFα) enhances matrix metalloproteinase-9 (MMP-9) gene expression and activity in fetal membranes, contributing to membrane weakening and rupture. We previously demonstrated that progestins attenuate TNFα-induced MMP-9 activity in a cytotrophoblast cell line. However, whether they have a similar effect in primary amnion and chorion cells of fetal membranes is unknown. In this study, we evaluated the effect of progestins on basal and TNFα-induced MMP-9 activity and gene expression in primary chorion and amnion cells harvested from the fetal membranes of term nonlaboring patients.
Methods: Primary amnion and chorion cells were isolated from fetal membranes obtained from term uncomplicated nonlaboring patients following elective cesarean delivery (n = 11). Confluent primary amnion and chorion cell cultures were both pretreated with vehicle (control), progesterone (P4), 17α-hydroxyprogesterone caproate (17P), or medroxyprogesterone acetate (MPA) at 10 M concentration for 6 hours followed by stimulation with TNFα at 10 ng/mL for an additional 24 hours. Cell cultures pretreated with the vehicle only served as the unstimulated control and the vehicle stimulated with TNFα served as the stimulated control. Both controls were assigned a value of 100 units. Cell culture medium was harvested for MMP-9 enzymatic activity quantification using gelatin zymography. Total RNA was extracted for quantifying MMP-9 gene expression using real-time quantitative PCR. Basal MMP-9 activity and gene expression data were normalized to the unstimulated control. TNFα-stimulated MMP-9 activity and gene expression were normalized to the stimulated control. The primary outcome was the effect of progestins on TNFα-induced MMP-9 enzymatic activity in term human primary amnion and chorion cells in vitro. Secondary outcomes included the effect of progestin therapy on TNFα-induced MMP-9 gene expression and on basal MMP-9 activity and gene expression in primary amnion and chorion cells in vitro.
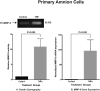
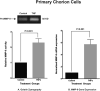
Results: Primary cells were harvested from 11 patients. Compared with the unstimulated control, TNFα increased MMP-9 activity (P = 0.005 versus control in primary amnion cells and P < 0.001 versus control in primary chorion cells) and MMP-9 gene expression (P = 0.030 versus control in primary amnion cells, P < 0.001 versus control in primary chorion cells). Compared with the unstimulated controls, MPA, but not P4 or 17P, reduced basal MMP-9 activity [mean difference (95% CI) -49.6 (-81.9, -17.3) units, P = 0.001] and gene expression [mean difference (95% CI) -53.4 (-105.9, -0.9) units, P = 0.045] in primary amnion cells. Compared with the stimulated control, MPA also reduced TNFα-induced MMP-9 activity [mean difference (95% CI) -69.0 (-91.8, -46.3) units, P < 0.001] and gene expression [mean difference (95% CI) -86.0 (-120.7, -51.3) units, P < 0.001] in primary amnion cells. Progestin pretreatment had no significant effect on basal or TNFα-induced MMP-9 activity and gene expression in primary chorion cells.
Conclusions: The inhibitory effect of MPA on both basal and TNFα-induced MMP-9 activity and gene expression in primary amnion cells demonstrate a possible mechanism by which progestins may prevent fetal membrane weakening leading to preterm premature rupture of membranes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
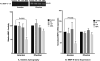
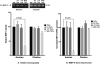
References
-
- Hamilton B, Martin J, Ventura S. National Vital Statistics Report. 3. Vol. 62. Hyattsville, MD: National Center for Health Statistics; 2013. Births: Preliminary Data for 2012. 2013. Available form http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_03.pdf) - PubMed
-
- Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Med. 2009;22:780–4. - PubMed
-
- Newman D, Paamoni-Keren O, Press F, Wiznitzer A, Mazor M, Sheiner E. Neonatal outcome in preterm deliveries between 23 and 27 weeks' gestation with and without preterm premature rupture of membranes. Arch Gynecol Obstet. 2009;280:7–11. - PubMed
-
- Fortunato SJ, Menon R, Lombardi SJ. Amniochorion gelatinase-gelatinase inhibitor imbalance in vitro: a possible infectious pathway to rupture. Obstet Gynecol. 2000;95:240–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

