Oxidative Transformation of Demethoxy- and Bisdemethoxycurcumin: Products, Mechanism of Formation, and Poisoning of Human Topoisomerase IIα
- PMID: 25806475
- PMCID: PMC4437832
- DOI: 10.1021/acs.chemrestox.5b00009
Oxidative Transformation of Demethoxy- and Bisdemethoxycurcumin: Products, Mechanism of Formation, and Poisoning of Human Topoisomerase IIα
Abstract
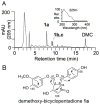
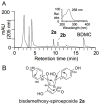
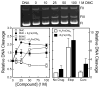
Extracts from the rhizome of the turmeric plant are widely consumed as anti-inflammatory dietary supplements. Turmeric extract contains the three curcuminoids, curcumin (≈80% relative abundance), demethoxycurcumin (DMC; ≈15%), and bisdemethoxycurcumin (BDMC; ≈5%). A distinct feature of pure curcumin is its instability at physiological pH, resulting in rapid autoxidation to a bicyclopentadione within 10-15 min. Here, we describe oxidative transformation of turmeric extract, DMC, and BDMC and the identification of their oxidation products using LC-MS and NMR analyses. DMC autoxidized over the course of 24 h to the expected bicyclopentadione diastereomers. BDMC was resistant to autoxidation, and oxidative transformation required catalysis by horseradish peroxidase and H2O2 or potassium ferricyanide. The product of BDMC oxidation was a stable spiroepoxide that was equivalent to a reaction intermediate in the autoxidation of curcumin. The ability of DMC and BDMC to poison recombinant human topoisomerase IIα was significantly increased in the presence of potassium ferricyanide, indicating that oxidative transformation was required to achieve full DNA cleavage activity. DMC and BDMC are less prone to autoxidation than curcumin and contribute to the enhanced stability of turmeric extract at physiological pH. Their oxidative metabolites may contribute to the biological effects of turmeric extract.
Figures
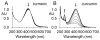
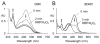
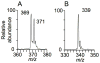
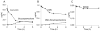
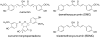
References
-
- Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin-from molecule to biological function. Angew Chem Int Ed Engl. 2012;51:5308–5332. - PubMed
-
- Norris L, Karmokar A, Howells L, Steward WP, Gescher A, Brown K. The role of cancer stem cells in the anti-carcinogenicity of curcumin. Mol Nutr Food Res. 2013;57:1630–1637. - PubMed
-
- Heger M, van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2014;66:222–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007628/GM/NIGMS NIH HHS/United States
- F31 AT007287/AT/NCCIH NIH HHS/United States
- GM033944/GM/NIGMS NIH HHS/United States
- AT006896/AT/NCCIH NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- 5P50CA095103/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT006896/AT/NCCIH NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- R03 CA159382/CA/NCI NIH HHS/United States
- CA159382/CA/NCI NIH HHS/United States
- P30DK058404/DK/NIDDK NIH HHS/United States
- R01 GM033944/GM/NIGMS NIH HHS/United States
- F31AT007287/AT/NCCIH NIH HHS/United States
- 2T32GM07628/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

