Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion
- PMID: 25806685
- PMCID: PMC4400582
- DOI: 10.7554/eLife.06557
Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion
Abstract
The role of specific phospholipids (PLs) in lipid transport has been difficult to assess due to an inability to selectively manipulate membrane composition in vivo. Here we show that the phospholipid remodeling enzyme lysophosphatidylcholine acyltransferase 3 (Lpcat3) is a critical determinant of triglyceride (TG) secretion due to its unique ability to catalyze the incorporation of arachidonate into membranes. Mice lacking Lpcat3 in the intestine fail to thrive during weaning and exhibit enterocyte lipid accumulation and reduced plasma TGs. Mice lacking Lpcat3 in the liver show reduced plasma TGs, hepatosteatosis, and secrete lipid-poor very low-density lipoprotein (VLDL) lacking arachidonoyl PLs. Mechanistic studies indicate that Lpcat3 activity impacts membrane lipid mobility in living cells, suggesting a biophysical basis for the requirement of arachidonoyl PLs in lipidating lipoprotein particles. These data identify Lpcat3 as a key factor in lipoprotein production and illustrate how manipulation of membrane composition can be used as a regulatory mechanism to control metabolic pathways.
Keywords: cell biology; human biology; lipoprotein; medicine; mouse; nuclear receptor; phospholipid.
Conflict of interest statement
SGY: Reviewing editor,
PT: Reviewing editor,
The other authors declare that no competing interests exist.
Figures


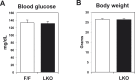
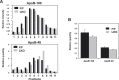
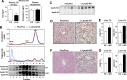
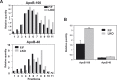

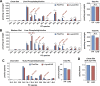


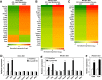
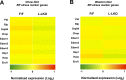
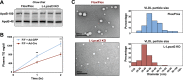

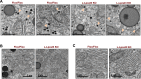
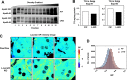

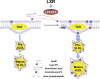
References
Publication types
MeSH terms
Substances
Grants and funding
- HL090553/HL/NHLBI NIH HHS/United States
- HL074214/HL/NHLBI NIH HHS/United States
- HL030568/HL/NHLBI NIH HHS/United States
- P01 HL090553/HL/NHLBI NIH HHS/United States
- GM076516/GM/NIGMS NIH HHS/United States
- R01 HL074214/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- Howard Hughes Medical Institute/United States
- P50 GM076516/GM/NIGMS NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- P41 GM103540/GM/NIGMS NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
- GM103540/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

