Phosphoenolpyruvate carboxykinase 1 gene (Pck1) displays parallel evolution between Old World and New World fruit bats
- PMID: 25807515
- PMCID: PMC4373879
- DOI: 10.1371/journal.pone.0118666
Phosphoenolpyruvate carboxykinase 1 gene (Pck1) displays parallel evolution between Old World and New World fruit bats
Abstract
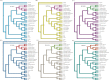
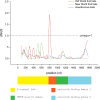
Bats are an ideal mammalian group for exploring adaptations to fasting due to their large variety of diets and because fasting is a regular part of their life cycle. Mammals fed on a carbohydrate-rich diet experience a rapid decrease in blood glucose levels during a fast, thus, the development of mechanisms to resist the consequences of regular fasts, experienced on a daily basis, must have been crucial in the evolution of frugivorous bats. Phosphoenolpyruvate carboxykinase 1 (PEPCK1, encoded by the Pck1 gene) is the rate-limiting enzyme in gluconeogenesis and is largely responsible for the maintenance of glucose homeostasis during fasting in fruit-eating bats. To test whether Pck1 has experienced adaptive evolution in frugivorous bats, we obtained Pck1 coding sequence from 20 species of bats, including five Old World fruit bats (OWFBs) (Pteropodidae) and two New World fruit bats (NWFBs) (Phyllostomidae). Our molecular evolutionary analyses of these sequences revealed that Pck1 was under purifying selection in both Old World and New World fruit bats with no evidence of positive selection detected in either ancestral branch leading to fruit bats. Interestingly, however, six specific amino acid substitutions were detected on the ancestral lineage of OWFBs. In addition, we found considerable evidence for parallel evolution, at the amino acid level, between the PEPCK1 sequences of Old World fruit bats and New World fruit bats. Test for parallel evolution showed that four parallel substitutions (Q276R, R503H, I558V and Q593R) were driven by natural selection. Our study provides evidence that Pck1 underwent parallel evolution between Old World and New World fruit bats, two lineages of mammals that feed on a carbohydrate-rich diet and experience regular periods of fasting as part of their life cycle.
Conflict of interest statement
Figures



Similar articles
-
Molecular Evolution of the Nuclear Factor (Erythroid-Derived 2)-Like 2 Gene Nrf2 in Old World Fruit Bats (Chiroptera: Pteropodidae).PLoS One. 2016 Jan 6;11(1):e0146274. doi: 10.1371/journal.pone.0146274. eCollection 2016. PLoS One. 2016. PMID: 26735303 Free PMC article.
-
The glycogen synthase 2 gene (Gys2) displays parallel evolution between Old World and New World fruit bats.J Mol Evol. 2014 Jan;78(1):66-74. doi: 10.1007/s00239-013-9600-1. Epub 2013 Nov 21. J Mol Evol. 2014. PMID: 24258790
-
Adaptive evolution in the glucose transporter 4 gene Slc2a4 in Old World fruit bats (family: Pteropodidae).PLoS One. 2012;7(4):e33197. doi: 10.1371/journal.pone.0033197. Epub 2012 Apr 6. PLoS One. 2012. PMID: 22493665 Free PMC article.
-
The neglected PCK1/glucagon (inter)action in nutrient homeostasis beyond gluconeogenesis: Disease pathogenesis and treatment.Mol Metab. 2025 Apr;94:102112. doi: 10.1016/j.molmet.2025.102112. Epub 2025 Feb 13. Mol Metab. 2025. PMID: 39954782 Free PMC article. Review.
-
PCK1 and PCK2 as candidate diabetes and obesity genes.Cell Biochem Biophys. 2007;48(2-3):89-95. doi: 10.1007/s12013-007-0025-6. Cell Biochem Biophys. 2007. PMID: 17709878 Review.
Cited by
-
Molecular Evolution of the Nuclear Factor (Erythroid-Derived 2)-Like 2 Gene Nrf2 in Old World Fruit Bats (Chiroptera: Pteropodidae).PLoS One. 2016 Jan 6;11(1):e0146274. doi: 10.1371/journal.pone.0146274. eCollection 2016. PLoS One. 2016. PMID: 26735303 Free PMC article.
-
Integrative single-cell characterization of a frugivorous and an insectivorous bat kidney and pancreas.Nat Commun. 2024 Jan 9;15(1):12. doi: 10.1038/s41467-023-44186-y. Nat Commun. 2024. PMID: 38195585 Free PMC article.
-
Dietary Diversification and Specialization in Neotropical Bats Facilitated by Early Molecular Evolution.Mol Biol Evol. 2021 Aug 23;38(9):3864-3883. doi: 10.1093/molbev/msab028. Mol Biol Evol. 2021. PMID: 34426843 Free PMC article.
-
Protective effect of phosphoenolpyruvate carboxykinase 1 on inflammation and fibrotic progression of IgA nephropathy.Ren Fail. 2025 Dec;47(1):2508297. doi: 10.1080/0886022X.2025.2508297. Epub 2025 May 29. Ren Fail. 2025. PMID: 40442892 Free PMC article.
References
-
- Boyle PJ, Shah SD, Cryer PE (1989) Insulin, glucagon, and catecholamines in prevention of hypoglycemia during fasting. Am J Physiol 256: E651–661. - PubMed
-
- Shrayyef MZ, Gerich JE (2010) Normal glucose homeostasis Principles of Diabetes Mellitus: Springer; pp. 19–35.
-
- Nordlie RC, Foster JD, Lange AJ (1999) Regulation of glucose production by the liver. Annu Rev Nutr 19: 379–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

