Tempering allorecognition to induce transplant tolerance with chemically modified apoptotic donor cells
- PMID: 25807873
- PMCID: PMC4439351
- DOI: 10.1111/ajt.13237
Tempering allorecognition to induce transplant tolerance with chemically modified apoptotic donor cells
Abstract
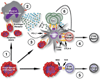
The development of organ transplantation as a therapy for end-stage organ failure is among the most significant achievements of 20th century medicine, but chronic rejection remains a barrier to achieving long-term success. Current therapeutic regimens consist of immunosuppressive drugs that are efficient at delaying rejection but are associated with significant risks such as opportunistic infections, toxicity, and malignancy. Thus, the induction of specific immune tolerance to transplant antigens is the coveted aim of researchers. The use of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (ECDI)-treated, autoantigen-coupled syngeneic leukocytes has been developed as a specific immunotherapy in preclinical models of autoimmunity and is currently in a phase II clinical trial for the treatment of multiple sclerosis. In this review, we discuss the use of allogeneic ECDI-treated apoptotic donor leukocytes (allo-ECDI-SP) as a strategy for inducing antigen-specific tolerance in allogeneic transplantation. Allo-ECDI-SP therapy induces long-term systemic immune tolerance to transplant antigens by subverting alloimmune recognition and exploiting apoptotic cell uptake pathways to recapitulate innate mechanisms of peripheral tolerance. Lastly, we discuss potential indications and challenges for transitioning allo-ECDI-SP therapy into clinical practice.
Keywords: Basic (laboratory) research/science; T cell biology; cell death: apoptosis; dendritic cell; immunobiology; islet transplantation; tolerance; translational research/science.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
References
-
- Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

