Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline
- PMID: 25808087
- PMCID: PMC4375780
- DOI: 10.1038/ncomms7606
Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline
Abstract
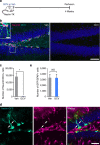
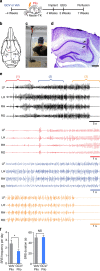
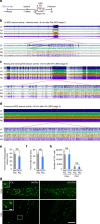
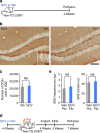
Acute seizures after a severe brain insult can often lead to epilepsy and cognitive impairment. Aberrant hippocampal neurogenesis follows the insult but the role of adult-generated neurons in the development of chronic seizures or associated cognitive deficits remains to be determined. Here we show that the ablation of adult neurogenesis before pilocarpine-induced acute seizures in mice leads to a reduction in chronic seizure frequency. We also show that ablation of neurogenesis normalizes epilepsy-associated cognitive deficits. Remarkably, the effect of ablating adult neurogenesis before acute seizures is long lasting as it suppresses chronic seizure frequency for nearly 1 year. These findings establish a key role of neurogenesis in chronic seizure development and associated memory impairment and suggest that targeting aberrant hippocampal neurogenesis may reduce recurrent seizures and restore cognitive function following a pro-epileptic brain insult.
Figures
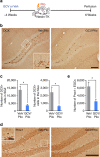
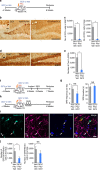

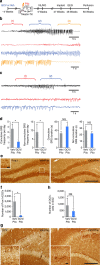
References
-
- Chang B. S. & Lowenstein D. H. Epilepsy. New Engl. J. Med. 349, 1257–1266 (2003) . - PubMed
-
- Engel J. Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 7, 340–352 (2001) . - PubMed
-
- Pitkanen A. & Sutula T. P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 1, 173–181 (2002) . - PubMed
-
- Eriksson P. S. et al. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 (1998) . - PubMed
-
- Ming G. L. & Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 (2005) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038572/NS/NINDS NIH HHS/United States
- R01 DA016765/DA/NIDA NIH HHS/United States
- R21 MH094715/MH/NIMH NIH HHS/United States
- R01NS081203/NS/NINDS NIH HHS/United States
- R01DA016765/DA/NIDA NIH HHS/United States
- K02AG041815/AG/NIA NIH HHS/United States
- R01 AG032383/AG/NIA NIH HHS/United States
- 5T32GM083831-05/GM/NIGMS NIH HHS/United States
- T32 GM083831/GM/NIGMS NIH HHS/United States
- R01AG032383/AG/NIA NIH HHS/United States
- T32 HL007360/HL/NHLBI NIH HHS/United States
- 5T32HL007360-34/HL/NHLBI NIH HHS/United States
- R01 NS081203/NS/NINDS NIH HHS/United States
- R01 NS076775/NS/NINDS NIH HHS/United States
- R01NS076775/NS/NINDS NIH HHS/United States
- R01NS038572/NS/NINDS NIH HHS/United States
- K02 AG041815/AG/NIA NIH HHS/United States
- K02 DA023555/DA/NIDA NIH HHS/United States
- K02DA023555/DA/NIDA NIH HHS/United States
- R01 NS048192/NS/NINDS NIH HHS/United States
- R21 DA023701/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

