Intensified antineoplastic effect by combining an HDAC-inhibitor, an mTOR-inhibitor and low dosed interferon alpha in prostate cancer cells
- PMID: 25808196
- PMCID: PMC4549030
- DOI: 10.1111/jcmm.12583
Intensified antineoplastic effect by combining an HDAC-inhibitor, an mTOR-inhibitor and low dosed interferon alpha in prostate cancer cells
Abstract
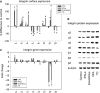
A significant proportion of men diagnosed with prostate cancer (PCa) eventually develop metastatic disease, which progresses to castration resistance, despite initial response to androgen deprivation. As anticancer therapy has become increasingly effective, acquired drug resistance has emerged, limiting efficacy. Combination treatment, utilizing different drug classes, exemplifies a possible strategy to foil resistance development. The effects of the triple application of the histone deacetylase (HDAC) inhibitor valproic acid (VPA), the mammalian target of rapamycin inhibitor everolimus and low dosed interferon alpha (IFNα) on PCa cell growth and dissemination capacity were investigated. For that purpose, the human PCa cell lines, PC-3, DU-145 and LNCaP were treated with the combined regimen or separate single agents. Cell growth was investigated by the MTT dye reduction assay. Flow cytometry served to analyse cell cycle progression. Adhesion to vascular endothelium or immobilized collagen, fibronectin and laminin was quantified. Migration and invasion characteristics were determined by the modified Boyden chamber assay. Integrin α and β subtypes were investigated by flow cytometry, western blotting and RT-PCR. Integrin related signalling, Epidermal Growth Factor Receptor (EGFr), Akt, p70S6kinase and extracellular signal-regulated kinases (ERK)1/2 activation were also assessed. The triple application of VPA, everolimus and low dosed IFNα blocked tumour cell growth and dissemination significantly better than any agent alone. Antitumour effects were associated with pronounced alteration in the cell cycle machinery, intracellular signalling and integrin expression profile. Combining VPA, everolimus and low dosed IFNα might be a promising option to counteract resistance development and improve outcome in PCa patients.
Keywords: combination therapy; everolimus; interferon alpha; prostate cancer; valproic acid.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. - PubMed
-
- MacVicar GR, Hussain MH. Emerging therapies in metastatic castration-sensitive and castration-resistant prostate cancer. Curr Opin Oncol. 2013;25:252–60. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. - PubMed
-
- Beltran H, Beer TM, Carducci MA, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60:279–90. - PubMed
-
- Sridhar SS, Freedland SJ, Gleave ME, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65:289–99. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

