Pregnancy outcome after TNF-α inhibitor therapy during the first trimester: a prospective multicentre cohort study
- PMID: 25808588
- PMCID: PMC4594709
- DOI: 10.1111/bcp.12642
Pregnancy outcome after TNF-α inhibitor therapy during the first trimester: a prospective multicentre cohort study
Abstract
Aims: TNF-α inhibitors are considered relatively safe in pregnancy but experience is still limited. The aim of this study was to evaluate the risk of major birth defects, spontaneous abortion, preterm birth and reduced birth weight after first trimester exposure to TNF-α inhibitors.
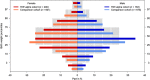
Methods: Pregnancy outcomes of women on adalimumab, infliximab, etanercept, certolizumab pegol or golimumab were evaluated in a prospective observational cohort study and compared with outcomes of a non-exposed random sample. The samples were drawn from pregnancies identified by institutes collaborating in the European Network of Teratology Information Services.
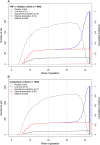
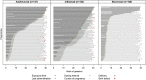
Results: In total, 495 exposed and 1532 comparison pregnancies were contributed from nine countries. The risk of major birth defects was increased in the exposed (5.0%) compared with the non-exposed group (1.5%; adjusted odds ratio (ORadj ) 2.2, 95% CI 1.0, 4.8). The risk of preterm birth was increased (17.6%; ORadj 1.69, 95% CI 1.1, 2.5), but not the risk of spontaneous abortion (16.2%; adjusted hazard ratio [HRadj ] 1.06, 95% CI 0.7, 1.7). Birth weights adjusted for gestational age and sex were significantly lower in the exposed group compared to the non-exposed cohort (P = 0.02). As a diseased comparison group was not possible to ascertain, the influence of disease and treatment on birth weight and preterm birth could not be differentiated.
Conclusions: TNF-α inhibitors may carry a risk of adverse pregnancy outcome of moderate clinical relevance. Considering the impact of insufficiently controlled autoimmune disease on the mother and the unborn child, TNF-α inhibitors may nevertheless be a treatment option in women with severe disease refractory to established immunomodulatory drugs.
Keywords: TNF-α inhibitors; birth defects; birth weight; malformations; pregnancy outcome.
© 2015 The British Pharmacological Society.
Figures
References
-
- Nielsen OH, Loftus EV, Jr, Jess T. Safety of TNF-α inhibitors during IBD pregnancy: a systematic review. BMC Med. 2013;11:174. . doi: 10.1186/1741-7015-11-174. - DOI - PMC - PubMed
-
- Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–7. - PMC - PubMed
-
- Narula N, Al-Dabbagh R, Dhillon A, Sands BE, Marshall JK. Anti-TNFalpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:1862–9. - PubMed
-
- Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603–5. - PubMed
-
- Chambers CD, Johnson DL. Emerging data on the use of anti-tumor necrosis factor-alpha medications in pregnancy. Birth Defects Res A Clin Mol Teratol. 2012;94:607–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

