Inactivation of HDAC1 or HDAC2 induces gamma globin expression without altering cell cycle or proliferation
- PMID: 25808664
- PMCID: PMC4909492
- DOI: 10.1002/ajh.24019
Inactivation of HDAC1 or HDAC2 induces gamma globin expression without altering cell cycle or proliferation
Abstract
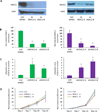
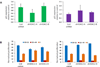
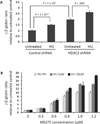
Other than hydroxyurea, no pharmacologic agents are clinically available for fetal hemoglobin (HbF) induction in sickle cell disease (SCD). An optimal candidate would induce HbF without causing cell cycle inhibition and would act independently of hydroxyurea in order to yield additional HbF induction when combined. We explored whether inhibition of histone deacetylase (HDAC) 1 or HDAC2 could achieve these goals. In human erythroid progenitor cells, shRNA knockdown of the HDAC1 or HDAC2 genes induced gamma globin, without altering cellular proliferation in vitro, and without altering cell cycle phase. Treatment with hydroxyurea in combination with HDAC2 knockdown yielded a further increase in gamma globin expression. Additionally, when CD34+ cells were treated with both hydroxyurea and MS-275 (an inhibitor of HDAC 1, 2, and 3), an additive induction of relative gamma globin expression was achieved. Our findings support further clinical investigation of HDAC inhibitors in combination with hydroxyurea in SCD patients.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. - PubMed
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. - PubMed
-
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
