Perspectives on biologically active camptothecin derivatives
- PMID: 25808858
- PMCID: PMC4465867
- DOI: 10.1002/med.21342
Perspectives on biologically active camptothecin derivatives
Abstract
Camptothecins (CPTs) are cytotoxic natural alkaloids that specifically target DNA topoisomerase I. Research on CPTs has undergone a significant evolution from the initial discovery of CPT in the late 1960s through the study of synthetic small-molecule derivatives to investigation of macromolecular constructs and formulations. Over the past years, intensive medicinal chemistry efforts have generated numerous CPT derivatives. Three derivatives, topotecan, irinotecan, and belotecan, are currently prescribed as anticancer drugs, and several related compounds are now in clinical trials. Interest in other biological effects, besides anticancer activity, of CPTs is also growing exponentially, as indicated by the large number of publications on the subject during the last decades. Therefore, the main focus of the present review is to provide an ample but condensed overview on various biological activities of CPT derivatives, in addition to continued up-to-date coverage of anticancer effects.
Keywords: DNA topoisomerase I; biological activities; camptothecins; structure-activity relationship.
© 2015 Wiley Periodicals, Inc.
Figures

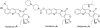
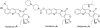

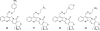

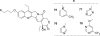



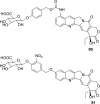



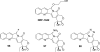


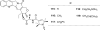

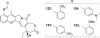

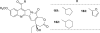
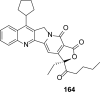

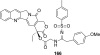
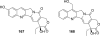

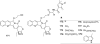

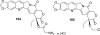

References
-
- Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888–90.
-
- Gottlieb JA, Guarino AM, Call JB, Oliverio VT, Block JB. Preliminary pharmacologic and clinical evaluation of camptothecin sodium (NSC-100880). Cancer Chemother Rep. 1970;54:461–79. - PubMed
-
- Muggia FM, Creaven PJ, Jansen HH, Cohen MN, Selawry DS. Phase I clinical trials of weekly and daily treatment with camptothecin (NSC 100880). Correlation with clinical studies. Cancer Chemother Rep. 1972;56:515–21. - PubMed
-
- Moertel CG, Schutt AJ, Reitemeier RJ, Hahn RG. Phase II study of camptothecin (NSC-100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother Rep. 1972;56:95–101. - PubMed
-
- Potmesil M, Kohn KW. DNA Topoisomerases in Cancer. Oxford University; New York: 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

