F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells
- PMID: 25809162
- PMCID: PMC4506886
- DOI: 10.1007/s00018-015-1890-6
F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells
Abstract
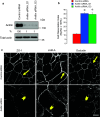
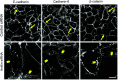
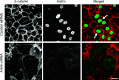
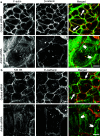
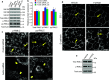
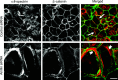
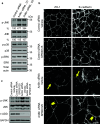
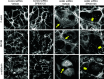
Tight junctions (TJ) and adherens junctions (AJ) are key morphological features of differentiated epithelial cells that regulate the integrity and permeability of tissue barriers. Structure and remodeling of epithelial junctions depends on their association with the underlying actomyosin cytoskeleton. Anillin is a unique scaffolding protein interacting with different cytoskeletal components, including actin filaments and myosin motors. Its role in the regulation of mammalian epithelial junctions remains unexplored. Downregulation of anillin expression in human prostate, colonic, and lung epithelial cells triggered AJ and TJ disassembly without altering the expression of junctional proteins. This junctional disassembly was accompanied by dramatic disorganization of the perijunctional actomyosin belt; while the general architecture of the actin cytoskeleton, and activation status of non-muscle myosin II, remained unchanged. Furthermore, loss of anillin disrupted the adducin-spectrin membrane skeleton at the areas of cell-cell contact, selectively decreased γ-adducin expression, and induced cytoplasmic aggregation of αII-spectrin. Anillin knockdown activated c-Jun N-terminal kinase (JNK), and JNK inhibition restored AJ and TJ integrity and cytoskeletal organization in anillin-depleted cells. These findings suggest a novel role for anillin in regulating intercellular adhesion in model human epithelia by mechanisms involving the suppression of JNK activity and controlling the assembly of the perijunctional cytoskeleton.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

