The HIV-1 antisense protein (ASP) induces CD8 T cell responses during chronic infection
- PMID: 25809376
- PMCID: PMC4335690
- DOI: 10.1186/s12977-015-0135-y
The HIV-1 antisense protein (ASP) induces CD8 T cell responses during chronic infection
Abstract
Background: CD8+ T cells recognize HIV-1 epitopes translated from a gene's primary reading frame (F1) and any one of its five alternative reading frames (ARFs) in the forward (F2, F3) or reverse (R1-3) directions. The 3' end of HIV-1's proviral coding strand contains a conserved sequence that is directly overlapping but antiparallel to the env gene (ARF R2) and encodes for a putative antisense HIV-1 protein called ASP. ASP expression has been demonstrated in vitro using HIV-transfected cell lines or infected cells. Although antibodies to ASP were previously detected in patient sera, T cell recognition of ASP-derived epitopes has not been evaluated. We therefore investigated the ex vivo and in vitro induction of ASP-specific T cell responses as a measure of immune recognition and protein expression during HIV-1 infection.
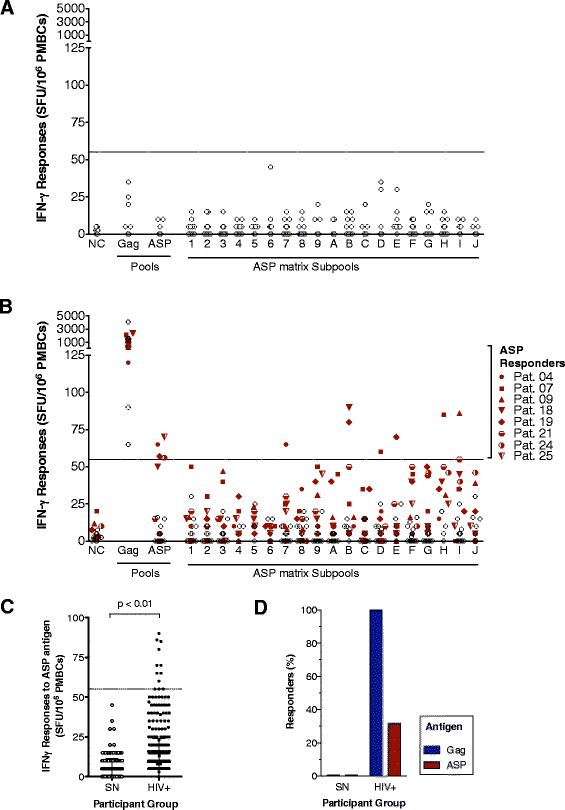
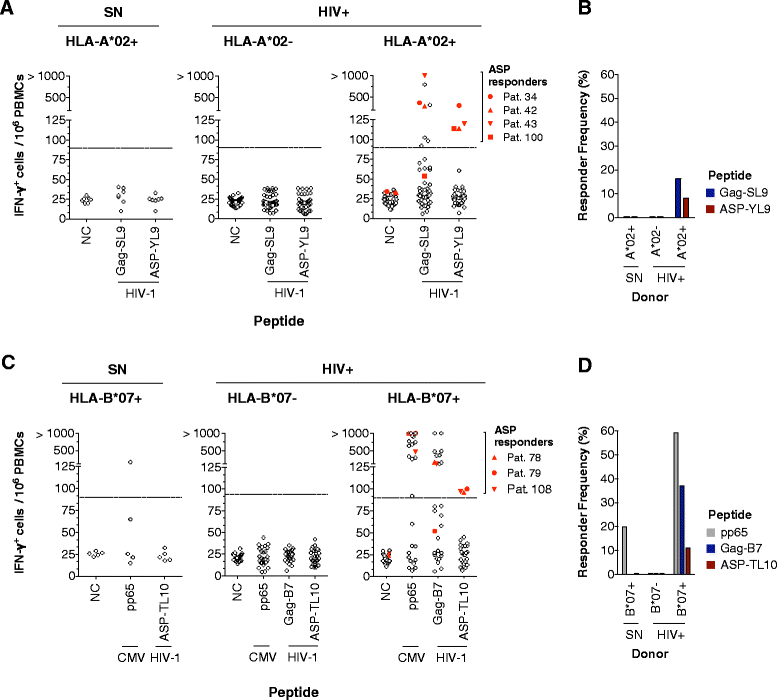
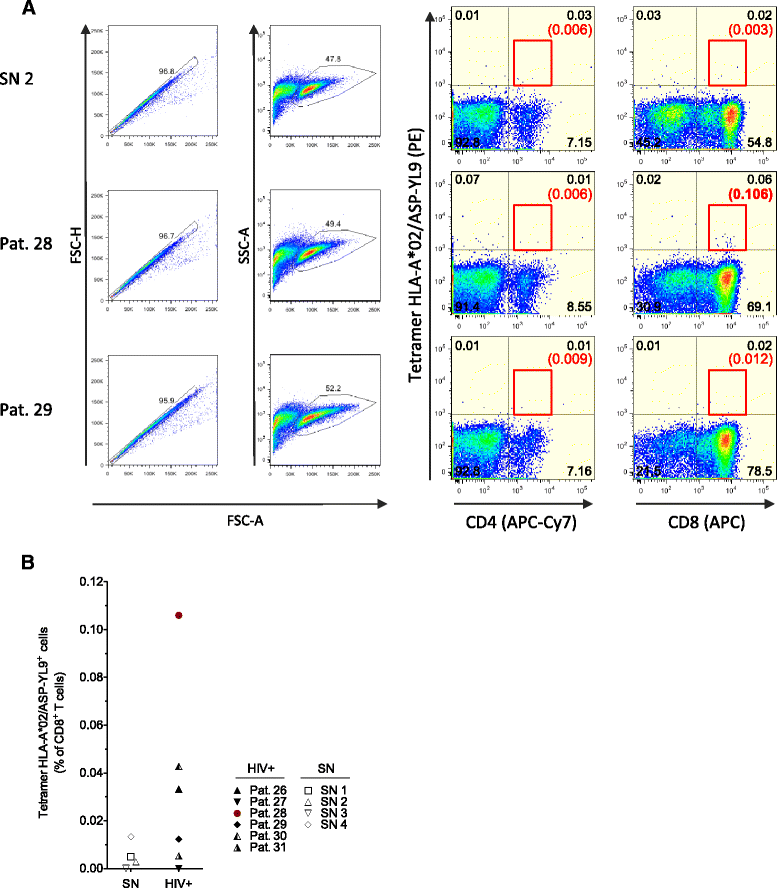
Results: A panel of overlapping peptides was initially designed from the full-length ASP sequence to perform a global assessment of T cell responses. Recognition of ASP-derived antigens was evaluated in an IFN-γELISpot assay using PBMCs from HIV-1 seropositive and seronegative individuals. Eight of 25 patients had positive responses to ASP antigens and none of the seronegative donors responded. As a complimentary approach, a second set of antigens was designed using HLA-I binding motifs and affinities. Two ASP-derived peptides with high predicted binding affinities for HLA-A*02 (ASP-YL9) and HLA-B*07 (ASP-TL10) were tested using PBMCs from HIV-1 seropositive and seronegative individuals who expressed the matching HLA-I-restricting allele. We found that HLA-I-restricted ASP peptides were only recognized by CD8+ T cells from patients with the relevant HLA-I and did not induce responses in any of the seronegative donors or patients who do not express the restrictive HLA alleles. Further, ASP-YL9-specific CD8+ T cells had functional profiles that were similar to a previously described HLA-A*02-restricted epitope (Gag-SL9). Specific recognition of ASP-YL9 by CD8+ T cells was also demonstrated by tetramer staining using cells from an HLA-A*02 HIV-infected patient.
Conclusion: Our results provide the first description of CD8+ T cell-mediated immune responses to ASP in HIV-1-infected patients, demonstrating that ASP is expressed during infection. Our identification of epitopes within ASP has implications for designing HIV vaccines.
Figures

References
-
- Wang YE, Li B, Carlson JM, Streeck H, Gladden AD, Goodman R, et al. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J Virol. 2009;83:1845–55. doi: 10.1128/JVI.01061-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

