The Non-catalytic B Subunit of Coagulation Factor XIII Accelerates Fibrin Cross-linking
- PMID: 25809477
- PMCID: PMC4424339
- DOI: 10.1074/jbc.M114.608570
The Non-catalytic B Subunit of Coagulation Factor XIII Accelerates Fibrin Cross-linking
Abstract
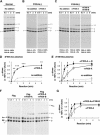
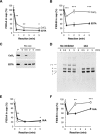
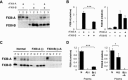
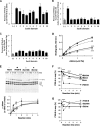
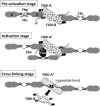
Covalent cross-linking of fibrin chains is required for stable blood clot formation, which is catalyzed by coagulation factor XIII (FXIII), a proenzyme of plasma transglutaminase consisting of catalytic A (FXIII-A) and non-catalytic B subunits (FXIII-B). Herein, we demonstrate that FXIII-B accelerates fibrin cross-linking. Depletion of FXIII-B from normal plasma supplemented with a physiological level of recombinant FXIII-A resulted in delayed fibrin cross-linking, reduced incorporation of FXIII-A into fibrin clots, and impaired activation peptide cleavage by thrombin; the addition of recombinant FXIII-B restored normal fibrin cross-linking, FXIII-A incorporation into fibrin clots, and activation peptide cleavage by thrombin. Immunoprecipitation with an anti-fibrinogen antibody revealed an interaction between the FXIII heterotetramer and fibrinogen mediated by FXIII-B and not FXIII-A. FXIII-B probably binds the γ-chain of fibrinogen with its D-domain, which is near the fibrin polymerization pockets, and dissociates from fibrin during or after cross-linking between γ-chains. Thus, FXIII-B plays important roles in the formation of a ternary complex between proenzyme FXIII, prosubstrate fibrinogen, and activator thrombin. Accordingly, congenital or acquired FXIII-B deficiency may result in increased bleeding tendency through impaired fibrin stabilization due to decreased FXIII-A activation by thrombin and secondary FXIII-A deficiency arising from enhanced circulatory clearance.
Keywords: Bleeding Disease; Blood; Coagulation Factor; Fibrin; Protein Cross-linking; Transglutaminase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Blombäck B., Hessel B., Hogg D., Therkildsen L. (1978) A two-step fibrinogen-fibrin transition in blood coagulation. Nature 275, 501–505 - PubMed
-
- Ichinose A., McMullen B. A., Fujikawa K., Davie E. W. (1986) Amino acid sequence of the B subunit of human coagulation factor XIII, a protein composed of ten repetitive segments. Biochemistry 25, 4633–4638 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

