Pathogenic mutations differentially affect the catalytic activities of the human B12-processing chaperone CblC and increase futile redox cycling
- PMID: 25809485
- PMCID: PMC4416844
- DOI: 10.1074/jbc.M115.637132
Pathogenic mutations differentially affect the catalytic activities of the human B12-processing chaperone CblC and increase futile redox cycling
Abstract
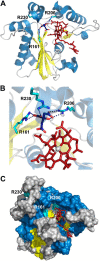
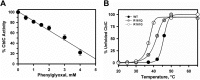
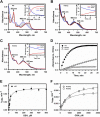
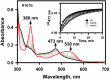
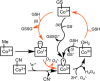
Human CblC catalyzes the elimination of the upper axial ligand in cobalamin or B12 derivatives entering the cell from circulation. This processing step is critical for assimilation of dietary cobalamin into the active cofactor forms that support the B12-dependent enzymes, methionine synthase and methylmalonyl-CoA mutase. Using a modified nitroreductase scaffold tailored to bind cobalamin and glutathione, CblC exhibits versatility in the mechanism by which it removes cyano versus alkyl ligands in cobalamin. In this study, we have characterized the effects of two pathogenic missense mutations at the same residue, R161G and R161Q, which are associated with early and late onset of the CblC disorder, respectively. We find that the R161Q and R161G CblC mutants display lower protein stability and decreased dealkylation but not decyanation activity, suggesting that cyanocobalamin might be therapeutically useful for patients carrying mutations at Arg-161. The mutant proteins also exhibit impaired glutathione binding. In the presence of physiologically relevant glutathione concentrations, stabilization of the cob(II)alamin derivative is observed, which occurs at the expense of increased oxidation of glutathione. Futile redox cycling, which is suppressed in wild-type human CblC, explains the reported increase in oxidative stress levels associated with the CblC disorder.
Keywords: Adenosylcobalamin (AdoCbl); Chaperone; Cofactor; Enzyme Kinetics; Oxidation-Reduction (Redox); Trafficking.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Banerjee R., Ragsdale S. W. (2003) The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 72, 209–247 - PubMed
-
- Banerjee R. (2001) Radical peregrinations catalyzed by coenzyme B12-dependent enzymes. Biochemistry 40, 6191–6198 - PubMed
-
- Watkins D., Rosenblatt D. S. (2012) Update and new concepts in vitamin responsive disorders of folate transport and metabolism. J. Inherit. Metab. Dis. 35, 665–670 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

