The regulation of neuronal mitochondrial metabolism by calcium
- PMID: 25809592
- PMCID: PMC4560577
- DOI: 10.1113/JP270254
The regulation of neuronal mitochondrial metabolism by calcium
Abstract
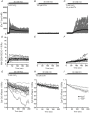
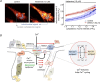
Calcium signalling is fundamental to the function of the nervous system, in association with changes in ionic gradients across the membrane. Although restoring ionic gradients is energetically costly, a rise in intracellular Ca(2+) acts through multiple pathways to increase ATP synthesis, matching energy supply to demand. Increasing cytosolic Ca(2+) stimulates metabolite transfer across the inner mitochondrial membrane through activation of Ca(2+) -regulated mitochondrial carriers, whereas an increase in matrix Ca(2+) stimulates the citric acid cycle and ATP synthase. The aspartate-glutamate exchanger Aralar/AGC1 (Slc25a12), a component of the malate-aspartate shuttle (MAS), is stimulated by modest increases in cytosolic Ca(2+) and upregulates respiration in cortical neurons by enhancing pyruvate supply into mitochondria. Failure to increase respiration in response to small (carbachol) and moderate (K(+) -depolarization) workloads and blunted stimulation of respiration in response to high workloads (veratridine) in Aralar/AGC1 knockout neurons reflect impaired MAS activity and limited mitochondrial pyruvate supply. In response to large workloads (veratridine), acute stimulation of respiration occurs in the absence of MAS through Ca(2+) influx through the mitochondrial calcium uniporter (MCU) and a rise in matrix [Ca(2+) ]. Although the physiological importance of the MCU complex in work-induced stimulation of respiration of CNS neurons is not yet clarified, abnormal mitochondrial Ca(2+) signalling causes pathology. Indeed, loss of function mutations in MICU1, a regulator of MCU complex, are associated with neuromuscular disease. In patient-derived MICU1 deficient fibroblasts, resting matrix Ca(2+) is increased and mitochondria fragmented. Thus, the fine tuning of Ca(2+) signals plays a key role in shaping mitochondrial bioenergetics.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



References
-
- Amigo I, Traba J, Gonzalez-Barroso MM, Rueda CB, Fernandez M, Rial E, Sanchez A, Satrustegui J, del Arco A. Glucagon regulation of oxidative phosphorylation requires an increase in matrix adenine nucleotide content through Ca2+-activation of the mitochondrial ATP-Mg/Pi carrier SCaMC-3. J Biol Chem. 2013;288:7791–7802. - PMC - PubMed
-
- Aprille JR. Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role. FASEB J. 1988;2:2547–2556. - PubMed
-
- Aprille JR. Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. J Bioenerg Biomembr. 1993;25:473–481. - PubMed
-
- Asimakis GK, Aprille JR. In vitro alteration of the size of the liver mitochondrial adenine nucleotide pool: correlation with respiratory functions. Arch Biochem Biophys. 1980;203:307–316. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

