An estrogen-induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations
- PMID: 25809780
- PMCID: PMC4529342
- DOI: 10.1002/cam4.445
An estrogen-induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations
Abstract
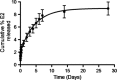
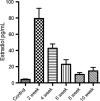
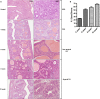
Endometrial hyperplasia (EH) is a condition originating from uterine endometrial glands undergoing disordered proliferation including the risk to progress to endometrial adenocarcinoma. In recent years, a steady increase in EH cases among younger women of reproductive age accentuates the demand of therapeutic alternatives, which emphasizes that an improved disease model for therapeutic agents evaluation is concurrently desired. Here, a new hormone-induced EH mouse model was developed using a subcutaneous estradiol (E2)-sustained releasing pellet, which elevates the serum E2 level in mice, closely mimicking the effect known as estrogen dominance with underlying, pathological E2 levels in patients. The onset and progression of EH generated within this model recapitulate a clinically relevant, pathological transformation, beginning with disordered proliferation developing to simple EH, advancing to atypical EH, and then progressing to precancerous stages, all following a chronologic manner. Although a general increase in nuclear progesterone receptor (PR) expression occurred after E2 expression, a total loss in PR was noted in some endometrial glands as disease advanced to simple EH. Furthermore, estrogen receptor (ER) expression in the nucleus of endometrial cells was reduced in disordered proliferation and increased when EH progressed to atypical EH and precancerous stages. This EH model also resembles other pathological patterns found in human disease such as leukocytic infiltration, genetic aberrations in β-catenin, and joint phosphatase and tensin homolog/paired box gene 2 (PTEN/PAX2) silencing. In summary, this new and comprehensively characterized EH model is cost-effective, easily reproducible, and may serve as a tool for preclinical testing of therapeutic agents and facilitate further investigation of EH.
Keywords: Endometrial cancer; endometrial hyperplasia; estrogen; mouse model.
© 2015 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures



References
-
- Siegel R, Ma J, Zou Z. Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. - PubMed
-
- Lacey JV. Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009;63:39–44. - PubMed
-
- Schlaen I, Bocanera R. Figueroa-Casas P. Endometrial cancer and its precursors: a comparison of histological and clinical features. Maturitas. 1986;8:335–44. - PubMed
-
- Kirschner MA, Schneider G, Ertel NH. Worton E. Obesity, androgens, estrogens, and cancer risk. Cancer Res. 1982;42(Suppl. 8):3281s–3285s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

