The conserved endoribonuclease YbeY is required for chloroplast ribosomal RNA processing in Arabidopsis
- PMID: 25810095
- PMCID: PMC4424013
- DOI: 10.1104/pp.114.255000
The conserved endoribonuclease YbeY is required for chloroplast ribosomal RNA processing in Arabidopsis
Abstract
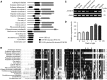
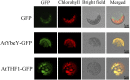
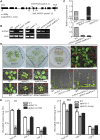
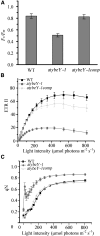
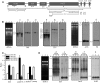
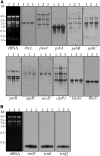
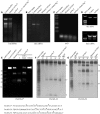
Maturation of chloroplast ribosomal RNAs (rRNAs) comprises several endoribonucleolytic and exoribonucleolytic processing steps. However, little is known about the specific enzymes involved and the cleavage steps they catalyze. Here, we report the functional characterization of the single Arabidopsis (Arabidopsis thaliana) gene encoding a putative YbeY endoribonuclease. AtYbeY null mutants are seedling lethal, indicating that AtYbeY function is essential for plant growth. Knockdown plants display slow growth and show pale-green leaves. Physiological and ultrastructural analyses of atybeY mutants revealed impaired photosynthesis and defective chloroplast development. Fluorescent microcopy analysis showed that, when fused with the green fluorescence protein, AtYbeY is localized in chloroplasts. Immunoblot and RNA gel-blot assays revealed that the levels of chloroplast-encoded subunits of photosynthetic complexes are reduced in atybeY mutants, but the corresponding transcripts accumulate normally. In addition, atybeY mutants display defective maturation of both the 5' and 3' ends of 16S, 23S, and 4.5S rRNAs as well as decreased accumulation of mature transcripts from the transfer RNA genes contained in the chloroplast rRNA operon. Consequently, mutant plants show a severe deficiency in ribosome biogenesis, which, in turn, results in impaired plastid translational activity. Furthermore, biochemical assays show that recombinant AtYbeY is able to cleave chloroplast rRNAs as well as messenger RNAs and transfer RNAs in vitro. Taken together, our findings indicate that AtYbeY is a chloroplast-localized endoribonuclease that is required for chloroplast rRNA processing and thus for normal growth and development.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures




Similar articles
-
Ribosomal RNA processing and an RNase R family member in chloroplasts of Arabidopsis.Plant Mol Biol. 2004 Jul;55(4):595-606. doi: 10.1007/s11103-004-1507-1. Plant Mol Biol. 2004. PMID: 15604703
-
A Member of the Arabidopsis Mitochondrial Transcription Termination Factor Family Is Required for Maturation of Chloroplast Transfer RNAIle(GAU).Plant Physiol. 2015 Sep;169(1):627-46. doi: 10.1104/pp.15.00964. Epub 2015 Jul 7. Plant Physiol. 2015. PMID: 26152711 Free PMC article.
-
A nuclear-encoded chloroplast protein harboring a single CRM domain plays an important role in the Arabidopsis growth and stress response.BMC Plant Biol. 2014 Apr 16;14:98. doi: 10.1186/1471-2229-14-98. BMC Plant Biol. 2014. PMID: 24739417 Free PMC article.
-
Chloroplast ribosomes and protein synthesis.Microbiol Rev. 1994 Dec;58(4):700-54. doi: 10.1128/mr.58.4.700-754.1994. Microbiol Rev. 1994. PMID: 7854253 Free PMC article. Review.
-
Variegation mutants and mechanisms of chloroplast biogenesis.Plant Cell Environ. 2007 Mar;30(3):350-365. doi: 10.1111/j.1365-3040.2006.01630.x. Plant Cell Environ. 2007. PMID: 17263779 Review.
Cited by
-
Identification of YbeY-Protein Interactions Involved in 16S rRNA Maturation and Stress Regulation in Escherichia coli.mBio. 2016 Nov 8;7(6):e01785-16. doi: 10.1128/mBio.01785-16. mBio. 2016. PMID: 27834201 Free PMC article.
-
YbeY is required for ribosome small subunit assembly and tRNA processing in human mitochondria.Nucleic Acids Res. 2021 Jun 4;49(10):5798-5812. doi: 10.1093/nar/gkab404. Nucleic Acids Res. 2021. PMID: 34037799 Free PMC article.
-
Suppression of Drug Resistance Reveals a Genetic Mechanism of Metabolic Plasticity in Malaria Parasites.mBio. 2018 Nov 13;9(6):e01193-18. doi: 10.1128/mBio.01193-18. mBio. 2018. PMID: 30425143 Free PMC article.
-
How RNases Shape Mitochondrial Transcriptomes.Int J Mol Sci. 2022 May 30;23(11):6141. doi: 10.3390/ijms23116141. Int J Mol Sci. 2022. PMID: 35682820 Free PMC article. Review.
-
The Arabidopsis AAC Proteins CIL and CIA2 Are Sub-functionalized Paralogs Involved in Chloroplast Development.Front Plant Sci. 2021 Jun 7;12:681375. doi: 10.3389/fpls.2021.681375. eCollection 2021. Front Plant Sci. 2021. PMID: 34163512 Free PMC article.
References
-
- Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6: 317–326 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Bang WY, Chen J, Jeong IS, Kim SW, Kim CW, Jung HS, Lee KH, Kweon HS, Yoko I, Shiina T, et al. (2012) Functional characterization of ObgC in ribosome biogenesis during chloroplast development. Plant J 71: 122–134 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

