Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation
- PMID: 25810312
- PMCID: PMC4425354
- DOI: 10.1126/scitranslmed.aaa6853
Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation
Abstract
Although major advances have been made in solid organ and hematopoietic stem cell transplantation in the last 50 years, big challenges remain. This review outlines the current immunological limitations for hematopoietic stem cell and solid organ transplantation and discusses new immune-modulating therapies in preclinical development and in clinical trials that may allow these obstacles to be overcome.
Copyright © 2015, American Association for the Advancement of Science.
Figures
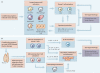
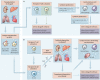
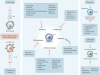
References
-
- Pasquini M, Wang Z. “Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2014,”. 2014 Available at http://www.cibmtr.org.
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of Allogeneic Hematopoietic Stem Cell Transplantation. Annual Review of Immunology. 2007;25:139–170. - PubMed
Publication types
MeSH terms
Grants and funding
- UM1 AI109565/AI/NIAID NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- R01 AI037691/AI/NIAID NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 AI112613/AI/NIAID NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- T32 AI007313/AI/NIAID NIH HHS/United States
- F30 HL121873/HL/NHLBI NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

