Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients
- PMID: 25810336
- PMCID: PMC4452400
- DOI: 10.1161/CIRCULATIONAHA.114.013698
Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients
Abstract
Background: Brugada syndrome (BrS) is a highly arrhythmogenic cardiac disorder, associated with an increased incidence of sudden death. Its arrhythmogenic substrate in the intact human heart remains ill-defined.
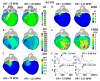
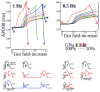
Methods and results: Using noninvasive ECG imaging, we studied 25 BrS patients to characterize the electrophysiological substrate and 6 patients with right bundle-branch block for comparison. Seven healthy subjects provided control data. Abnormal substrate was observed exclusively in the right ventricular outflow tract with the following properties (in comparison with healthy controls; P<0.005): (1) ST-segment elevation and inverted T wave of unipolar electrograms (2.21±0.67 versus 0 mV); (2) delayed right ventricular outflow tract activation (82±18 versus 37±11 ms); (3) low-amplitude (0.47±0.16 versus 3.74±1.60 mV) and fractionated electrograms, suggesting slow discontinuous conduction; (4) prolonged recovery time (381±30 versus 311±34 ms) and activation-recovery intervals (318±32 versus 241±27 ms), indicating delayed repolarization; (5) steep repolarization gradients (Δrecovery time/Δx=96±28 versus 7±6 ms/cm, Δactivation-recovery interval/Δx=105±24 versus 7±5 ms/cm) at right ventricular outflow tract borders. With increased heart rate in 6 BrS patients, reduced ST-segment elevation and increased fractionation were observed. Unlike BrS, right bundle-branch block had delayed activation in the entire right ventricle, without ST-segment elevation, fractionation, or repolarization abnormalities on electrograms.
Conclusions: The results indicate that both slow discontinuous conduction and steep dispersion of repolarization are present in the right ventricular outflow tract of BrS patients. ECG imaging could differentiate between BrS and right bundle-branch block.
Keywords: Brugada syndrome; electrocardiography; electrophysiology.
© 2015 American Heart Association, Inc.
Figures



References
-
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Riera ARP, Shimizu W, Schulze-Bahr E, Tan HL, Wilde AA. Brugada syndrome: Report of the second consensus conference endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. - PubMed
-
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. - PubMed
-
- Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Ann Med. 2004;36:5–14. - PubMed
-
- Aiba T, Shimizu W, Hidaka I, Uemura K, Noda T, Zheng C, Kamiya A, Inagaki M, Sugimachi M, Sunagawa K. Cellular Basis for Trigger and Maintenance of Ventricular Fibrillation in the Brugada Syndrome ModelHigh-Resolution Optical Mapping Study. J Am Coll Cardiol. 2006;47:2074–2085. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

