Brain-wide analysis of electrophysiological diversity yields novel categorization of mammalian neuron types
- PMID: 25810482
- PMCID: PMC4455486
- DOI: 10.1152/jn.00237.2015
Brain-wide analysis of electrophysiological diversity yields novel categorization of mammalian neuron types
Abstract
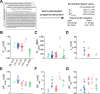
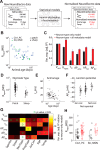
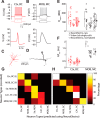
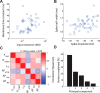
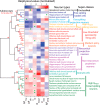
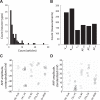
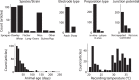
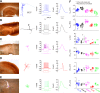
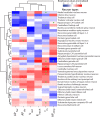
For decades, neurophysiologists have characterized the biophysical properties of a rich diversity of neuron types. However, identifying common features and computational roles shared across neuron types is made more difficult by inconsistent conventions for collecting and reporting biophysical data. Here, we leverage NeuroElectro, a literature-based database of electrophysiological properties (www.neuroelectro.org), to better understand neuronal diversity, both within and across neuron types, and the confounding influences of methodological variability. We show that experimental conditions (e.g., electrode types, recording temperatures, or animal age) can explain a substantial degree of the literature-reported biophysical variability observed within a neuron type. Critically, accounting for experimental metadata enables massive cross-study data normalization and reveals that electrophysiological data are far more reproducible across laboratories than previously appreciated. Using this normalized dataset, we find that neuron types throughout the brain cluster by biophysical properties into six to nine superclasses. These classes include intuitive clusters, such as fast-spiking basket cells, as well as previously unrecognized clusters, including a novel class of cortical and olfactory bulb interneurons that exhibit persistent activity at theta-band frequencies.
Keywords: databases; electrophysiology; intrinsic membrane properties; neuroinformatics; neuron biophysics; neuron diversity; text mining.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 215: 403–410, 1990. - PubMed
-
- Ambert KH, Cohen AM. Text-mining and neuroscience. Int Rev Neurobiol 103: 109–132, 2012. - PubMed
-
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, DeFelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9: 557–568, 2008. - PMC - PubMed
-
- Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci 27: 9247–9251, 2007. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

