Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction
- PMID: 25810557
- PMCID: PMC4442436
- DOI: 10.1128/JVI.00232-15
Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction
Abstract
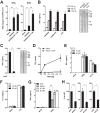
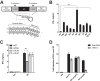
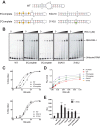
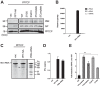
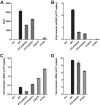
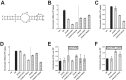
Retinoic acid-inducible gene I (RIG-I) is an important innate immune sensor that recognizes viral RNA in the cytoplasm. Its nonself recognition largely depends on the unique RNA structures imposed by viral RNA. The panhandle structure residing in the influenza A virus (IAV) genome, whose primary function is to serve as the viral promoter for transcription and replication, has been proposed to be a RIG-I agonist. However, this has never been proved experimentally. Here, we employed multiple approaches to determine if the IAV panhandle structure is directly involved in RIG-I activation and type I interferon (IFN) induction. First, in porcine alveolar macrophages, we demonstrated that the viral genomic coding region is dispensable for RIG-I-dependent IFN induction. Second, using in vitro-synthesized hairpin RNA, we showed that the IAV panhandle structure could directly bind to RIG-I and stimulate IFN production. Furthermore, we investigated the contributions of the wobble base pairs, mismatch, and unpaired nucleotides within the wild-type panhandle structure to RIG-I activation. Elimination of these destabilizing elements within the panhandle structure promoted RIG-I activation and IFN induction. Given the function of the panhandle structure as the viral promoter, we further monitored the promoter activity of these panhandle variants and found that viral replication was moderately affected, whereas viral transcription was impaired dramatically. In all, our results indicate that the IAV panhandle promoter region adopts a nucleotide composition that is optimal for balanced viral RNA synthesis and suboptimal for RIG-I activation.
Importance: The IAV genomic panhandle structure has been proposed to be an RIG-I agonist due to its partial complementarity; however, this has not been experimentally confirmed. Here, we provide direct evidence that the IAV panhandle structure is competent in, and sufficient for, RIG-I activation and IFN induction. By constructing panhandle variants with increased complementarity, we demonstrated that the wild-type panhandle structure could be modified to enhance RIG-I activation and IFN induction. These panhandle variants posed moderate influence on viral replication but dramatic impairment of viral transcription. These results indicate that the IAV panhandle promoter region adopts a nucleotide composition to achieve optimal balance of viral RNA synthesis and suboptimal RIG-I activation. Our results highlight the multifunctional role of the IAV panhandle promoter region in the virus life cycle and offer novel insights into the development of antiviral agents aiming to boost RIG-I signaling or virus attenuation by manipulating this conserved region.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi:10.1038/nature04734. - DOI - PubMed
-
- Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G. 2009. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31:25–34. doi:10.1016/j.immuni.2009.05.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

