In Vitro and In Vivo Anticancer Activity of Root Extracts of Sansevieria liberica Gerome and Labroy (Agavaceae)
- PMID: 25810744
- PMCID: PMC4354966
- DOI: 10.1155/2015/560404
In Vitro and In Vivo Anticancer Activity of Root Extracts of Sansevieria liberica Gerome and Labroy (Agavaceae)
Abstract
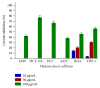
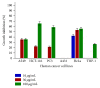
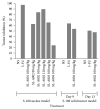
Introduction. Sansevieria liberica Gerome and Labroy (Agavaceae) is a perennial plant widely distributed in tropical Africa. Preparations of the plant are commonly used across Nigeria for the treatment of inflammatory conditions. Based on the fact that herbal medicine is a strong component of integrative medicine, this study was conducted to evaluate the anticancer activity of root extracts of Sansevieria liberica. Methods. Sulforhodamine B (SRB) in vitro cytotoxicity assay, Sarcoma-180 (S-180) ascites and solid tumor, and L1210 lymphoid leukemia in vivo models were used in this study. Results. SL-A002 (IC50 23 µg/mL with HeLa), SL-A003 (IC50 22 µg/mL with HCT-116), and SL-A004 (IC50 23 and 18 µg/mL with A549 and THP-1, resp.) demonstrated significant activity in the SRB cytotoxicity assay. Potency was highest with the following pairs of extract : cancer cell line: SL-A002 : HeLa (IC50 23 µg/mL), SL-A003 : HCT-116 (IC50 22 µg/mL), and SL-A004 : THP-1 (IC50 18 µg/mL). SL-A002 demonstrated significant dose-dependent antitumor activity in the Sarcoma-180 (S-180) ascites model with peak effect produced at the dose of 120 mg/kg (i.p.) with inhibition of 89.36% compared to 97.96% for 5-FU (20 mg/kg i.p.). The inhibition of tumor growth by SL-A002 in the S-180 solid tumor model was 47.40% compared to a value of 50.18% for 5-FU. SL-A002 was also significantly active in the L1210 lymphoid leukemia model with 158.33% increase in mean survival time, the same value for 5-FU. Conclusions. The hydroethanolic extract of Sansevieria liberica, SL-A002, possesses significant anticancer activity to warrant further extensive study to identify, isolate, and characterize the specific bioactive molecules responsible for the observed antitumor activity and the precise mechanism(s) of action.
Figures


References
-
- Rao M. R. P., Adagale U. R., Shetty A., Namjoshi P., Gaitonde P., Jain P. Cancer Immunotherapy. 2007. http://www.pharmainfo.net/reviews/cancer-immunotherapy.
-
- Mubeen M., Kini S. G. A review on the design and development of EGFR tyrosine kinase inhibitors in cancer therapy. International Journal of Therapeutic Applications. 2012;5:29–37.
-
- World Health Organization (WHO) The Global Burden of Disease: 2004 Update. Geneva, Switzerland: WHO; 2008.
LinkOut - more resources
Full Text Sources
Other Literature Sources

