SIRT3 and SIRT5 regulate the enzyme activity and cardiolipin binding of very long-chain acyl-CoA dehydrogenase
- PMID: 25811481
- PMCID: PMC4374878
- DOI: 10.1371/journal.pone.0122297
SIRT3 and SIRT5 regulate the enzyme activity and cardiolipin binding of very long-chain acyl-CoA dehydrogenase
Abstract
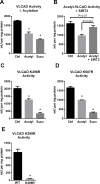
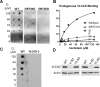
SIRT3 and SIRT5 have been shown to regulate mitochondrial fatty acid oxidation but the molecular mechanisms behind the regulation are lacking. Here, we demonstrate that SIRT3 and SIRT5 both target human very long-chain acyl-CoA dehydrogenase (VLCAD), a key fatty acid oxidation enzyme. SIRT3 deacetylates and SIRT5 desuccinylates K299 which serves to stabilize the essential FAD cofactor in the active site. Further, we show that VLCAD binds strongly to cardiolipin and isolated mitochondrial membranes via a domain near the C-terminus containing lysines K482, K492, and K507. Acetylation or succinylation of these residues eliminates binding of VLCAD to cardiolipin. SIRT3 deacetylates K507 while SIRT5 desuccinylates K482, K492, and K507. Sirtuin deacylation of recombinant VLCAD rescues membrane binding. Endogenous VLCAD from SIRT3 and SIRT5 knockout mouse liver shows reduced binding to cardiolipin. Thus, SIRT3 and SIRT5 promote fatty acid oxidation by converging upon VLCAD to promote its activity and membrane localization. Regulation of cardiolipin binding by reversible lysine acylation is a novel mechanism that is predicted to extrapolate to other metabolic proteins that localize to the inner mitochondrial membrane.
Conflict of interest statement
Figures



References
-
- Vockley J, Whiteman DA. Defects of mitochondrial beta-oxidation: a growing group of disorders. Neuromuscul Disord. 2002. Mar;12(3):235–46. PubMed PMID: . - PubMed
-
- Schrauwen-Hinderling VB, Roden M, Kooi ME, Hesselink MK, Schrauwen P. Muscular mitochondrial dysfunction and type 2 diabetes mellitus. Curr Opin Clin Nutr Metab Care. 2007. Nov;10(6):698–703. PubMed PMID: . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

