Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells
- PMID: 25811595
- PMCID: PMC4374860
- DOI: 10.1371/journal.pone.0123088
Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells
Abstract
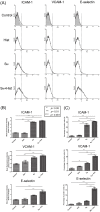
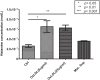
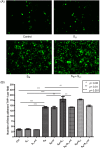
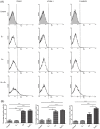
Oxidized low-density lipoprotein (OxLDL) is a risk factor for atherosclerosis, due to its role in endothelial dysfunction and foam cell formation. Tissue-resident cells such as macrophages and mast cells release inflammatory mediators upon activation that in turn cause endothelial activation and monocyte adhesion. Two of these mediators are tumor necrosis factor (TNF)-α, produced by macrophages, and histamine, produced by mast cells. Static and microfluidic flow experiments were conducted to determine the number of adherent monocytes on vascular endothelium activated by supernatants of oxLDL-treated macrophages and mast cells or directly by oxLDL. The expression of adhesion molecules on activated endothelial cells and the concentration of TNF-α and histamine in the supernatants were measured by flow cytometry and enzyme-linked immunosorbent assay, respectively. A low dose of oxLDL (8 μg/ml), below the threshold for the clinical presentation of coronary artery disease, was sufficient to activate both macrophages and mast cells and synergistically increase monocyte-endothelium adhesion via released TNF-α and histamine. The direct exposure of endothelial cells to a much higher dose of oxLDL (80 μg/ml) had less effect on monocyte adhesion than the indirect activation via oxLDL-treated macrophages and mast cells. The results of this work indicate that the co-activation of macrophages and mast cells by oxLDL is an important mechanism for the endothelial dysfunction and atherogenesis. The observed synergistic effect suggests that both macrophages and mast cells play a significant role in early stages of atherosclerosis. Allergic patients with a lipid-rich diet may be at high risk for cardiovascular events due to high concentration of low-density lipoprotein and histamine in arterial vessel walls.
Conflict of interest statement
Figures
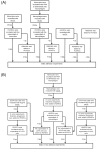
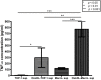
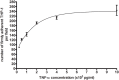
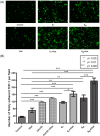

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

