Hydrogen sulfide regulates the colonic motility by inhibiting both L-type calcium channels and BKCa channels in smooth muscle cells of rat colon
- PMID: 25811907
- PMCID: PMC4374679
- DOI: 10.1371/journal.pone.0121331
Hydrogen sulfide regulates the colonic motility by inhibiting both L-type calcium channels and BKCa channels in smooth muscle cells of rat colon
Abstract
Objective: To examine the hypothesis that hydrogen sulfide (H2S) regulates the colonic motility by modulating both L-type voltage-dependent calcium channels and large conductance Ca2+-activated K+ (BKCa) channels.
Methods: Immunohistochemistry was performed on rat colonic samples to investigate the localization of the H2S-producing enzymes cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE). The contractions of proximal colonic smooth muscle were studied in an organ bath system. The whole-cell patch-clamp technique was used to record both L-type calcium currents (ICa,L) and BKCa currents in colonic smooth muscle cells (SMCs) isolated from male Wistar rats.
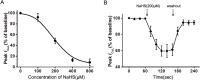
Results: Immunohistochemistry revealed the presence of CBS and CSE in mucosa, smooth muscle cells and myenteric neurons. The H2S donor NaHS inhibited spontaneous contractions of the longitudinal muscle and circular muscle strips in a dose-dependent manner, and the inhibitory effects were not blocked by tetrodotoxin. NaHS inhibited the peak ICa,L in colonic SMCs at a membrane potential of 0 mV. The current-voltage (I-V) relationship of L-type calcium channels was modified by NaHS, and the peak of the I-V curve was shifted to the right. NaHS (200 μΜ) evoked a significant rightward shift of the steady-state activation curve and inhibited the inactivation of L-type calcium channels. Furthermore, NaHS reversibly decreased the peak ICa,L in a dose-dependent manner. Likewise, BKCa channels were significantly inhibited by NaHS, and the addition of NaHS caused a time- and dose-dependent reduction in the BKCa current.
Conclusion: The relaxant effect of H2S on colonic muscle strips may be associated with the direct inhibition of H2S on L-type calcium channels. H2S may be involved in the regulation of calcium homeostasis in colonic SMCs of rat colon.
Conflict of interest statement
Figures







Similar articles
-
Mechanism of sodium hydrosulfide modulation of L-type calcium channels in rat colonic smooth muscle cells.Eur J Pharmacol. 2018 Jan 5;818:356-363. doi: 10.1016/j.ejphar.2017.11.002. Epub 2017 Nov 9. Eur J Pharmacol. 2018. PMID: 29104047
-
Hydrogen sulfide-induced enhancement of gastric fundus smooth muscle tone is mediated by voltage-dependent potassium and calcium channels in mice.World J Gastroenterol. 2015 Apr 28;21(16):4840-51. doi: 10.3748/wjg.v21.i16.4840. World J Gastroenterol. 2015. PMID: 25944997 Free PMC article.
-
[Effects of hydrogen sulfide on colonic contraction of rats and ion channel mechanisms].Zhonghua Yi Xue Za Zhi. 2015 Jun 9;95(22):1768-72. Zhonghua Yi Xue Za Zhi. 2015. PMID: 26704165 Chinese.
-
Role of hydrogen sulfide in secondary neuronal injury.Neurochem Int. 2014 Jan;64:37-47. doi: 10.1016/j.neuint.2013.11.002. Epub 2013 Nov 14. Neurochem Int. 2014. PMID: 24239876 Review.
-
New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant.Nutr Rev. 2004 Sep;62(9):348-53. doi: 10.1111/j.1753-4887.2004.tb00060.x. Nutr Rev. 2004. PMID: 15497768 Review.
Cited by
-
External Hemin as an Inhibitor of Mitochondrial Large-Conductance Calcium-Activated Potassium Channel Activity.Int J Mol Sci. 2022 Nov 2;23(21):13391. doi: 10.3390/ijms232113391. Int J Mol Sci. 2022. PMID: 36362175 Free PMC article.
-
Sulfur-containing gaseous signal molecules, ion channels and cardiovascular diseases.Br J Pharmacol. 2018 Apr;175(8):1114-1125. doi: 10.1111/bph.13829. Epub 2017 May 30. Br J Pharmacol. 2018. PMID: 28430359 Free PMC article. Review.
-
Hydrogen sulphide as a signalling molecule regulating physiopathological processes in gastrointestinal motility.Br J Pharmacol. 2017 Sep;174(17):2805-2817. doi: 10.1111/bph.13918. Epub 2017 Jul 27. Br J Pharmacol. 2017. PMID: 28631296 Free PMC article. Review.
-
Runchangningshen paste activates NLRP6 inflammasome-mediated autophagy to stimulate colonic mucin-2 secretion and modulates mucosal microbiota in functional constipation.World J Gastroenterol. 2025 Mar 7;31(9):102256. doi: 10.3748/wjg.v31.i9.102256. World J Gastroenterol. 2025. PMID: 40061589 Free PMC article.
-
Endogenous Hydrogen Sulfide Contributes to Tone Generation in Porcine Lower Esophageal Sphincter Via Na+/Ca2+ Exchanger.Cell Mol Gastroenterol Hepatol. 2017 Nov 22;5(3):209-221. doi: 10.1016/j.jcmgh.2017.11.004. eCollection 2018 Mar. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29379856 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

