Progress in human embryonic stem cell research in the United States between 2001 and 2010
- PMID: 25812114
- PMCID: PMC4374681
- DOI: 10.1371/journal.pone.0120052
Progress in human embryonic stem cell research in the United States between 2001 and 2010
Abstract
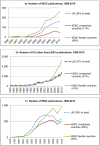
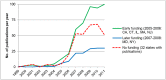
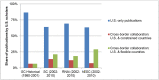
On August 9th, 2001, the federal government of the United States announced a policy restricting federal funds available for research on human embryonic stem cell (hESCs) out of concern for the "vast ethical mine fields" associated with the creation of embryos for research purposes. Until the policy was repealed on March 9th, 2009, no U.S. federal funds were available for research on hESCs extracted after August 9, 2001, and only limited federal funds were available for research on a subset of hESC lines that had previously been extracted. This paper analyzes how the 2001 U.S. federal funding restrictions influenced the quantity and geography of peer-reviewed journal publications on hESC. The primary finding is that the 2001 policy did not have a significant aggregate effect on hESC research in the U.S. After a brief lag in early 2000s, U.S. hESC research maintained pace with other areas of stem cell and genetic research. The policy had several other consequences. First, it was tied to increased hESC research funding within the U.S. at the state level, leading to concentration of related activities in a relatively small number of states. Second, it stimulated increased collaborative research between US-based scientists and those in countries with flexible policies toward hESC research (including Canada, the U.K., Israel, China, Spain, and South Korea). Third, it encouraged independent hESC research in countries without restrictions.
Conflict of interest statement
Figures
Similar articles
-
Emotion in political discourse: contrasting approaches to stem cell governance in the USA, UK, Israel and Germany.Regen Med. 2006 Nov;1(6):823-9. doi: 10.2217/17460751.1.6.823. Regen Med. 2006. PMID: 17465763
-
Human embryonic stem cell research and Proposition 71. Reflections on California’s response to federal policy.Politics Life Sci. 2010 Sep;29(2):73-95. doi: 10.2990/29_2_73. Politics Life Sci. 2010. PMID: 21761982
-
Transcriptional landscape changes during human embryonic stem cell derivation.Mol Hum Reprod. 2018 Nov 1;24(11):543-555. doi: 10.1093/molehr/gay039. Mol Hum Reprod. 2018. PMID: 30239859
-
Rethink the patentability of human embryonic stem cell research findings: Relaxation based on benefit weighing.Stem Cell Reports. 2021 Aug 10;16(8):1868-1873. doi: 10.1016/j.stemcr.2021.07.005. Stem Cell Reports. 2021. PMID: 34380021 Free PMC article. Review.
-
Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems.Reprod Biol Endocrinol. 2015 Feb 22;13:9. doi: 10.1186/s12958-015-0005-4. Reprod Biol Endocrinol. 2015. PMID: 25890180 Free PMC article. Review.
Cited by
-
Work-Education Mismatch: An Endogenous Theory of Professionalization.Eur J Oper Res. 2017 Sep 16;261(3):1085-1097. doi: 10.1016/j.ejor.2017.02.041. Epub 2017 Mar 8. Eur J Oper Res. 2017. PMID: 28713195 Free PMC article.
-
Media portrayal of ethical and social issues in brain organoid research.Philos Ethics Humanit Med. 2022 Apr 13;17(1):8. doi: 10.1186/s13010-022-00119-z. Philos Ethics Humanit Med. 2022. PMID: 35414094 Free PMC article.
-
Voting on Embryonic Stem Cell Research: Citizens More Supportive than Politicians.PLoS One. 2017 Jan 26;12(1):e0170656. doi: 10.1371/journal.pone.0170656. eCollection 2017. PLoS One. 2017. PMID: 28125626 Free PMC article.
-
Recent trends in the U.S. Behavioral and Social Sciences Research (BSSR) workforce.PLoS One. 2017 Feb 6;12(2):e0170887. doi: 10.1371/journal.pone.0170887. eCollection 2017. PLoS One. 2017. PMID: 28166252 Free PMC article.
References
-
- Bush G (2001), Speech on Stem Cells at http://georgewbush-whitehouse.archives.gov/news/releases/2001/08/2001080...
-
- For examples, see Owen-Smith J, McCormick J (2006) An international gap in human ES cell research. Nature Biotechnology 24: 391–392; Levine A. (2008) Identifying Under- and over-performing countries in research related to human embryonic stem cells. Cell Stem Cell 2, 521–524; Löser P., Schirm J., Guhr A., Wobus A., Kurtz A. (2010) Human embryonic stem cell lines and their use in international research. Stem Cells 28, 240–246. - PMC - PubMed
-
- Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, et al. (1998) Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 282: 5391, pp. 1145–1147. - PubMed
-
- Burgin E (2010) ‘Human embryonic stem cell research and Proposition 71: Reflections on California’s response to federal policy,’ Politics the Life Sciences, 29(2), pp. 73–95. doi: 10.2990/29_2_73 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

