Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation
- PMID: 25812521
- PMCID: PMC4886837
- DOI: 10.1038/ncb3138
Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation
Abstract
Primitive streak formation in the chick embryo involves large-scale highly coordinated flows of more than 100,000 cells in the epiblast. These large-scale tissue flows and deformations can be correlated with specific anisotropic cell behaviours in the forming mesendoderm through a combination of light-sheet microscopy and computational analysis. Relevant behaviours include apical contraction, elongation along the apical-basal axis followed by ingression, and asynchronous directional cell intercalation of small groups of mesendoderm cells. Cell intercalation is associated with sequential, directional contraction of apical junctions, the onset, localization and direction of which correlate strongly with the appearance of active myosin II cables in aligned apical junctions in neighbouring cells. Use of class specific myosin inhibitors and gene-specific knockdown shows that apical contraction and intercalation are myosin II dependent and also reveal critical roles for myosin I and myosin V family members in the assembly of junctional myosin II cables.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

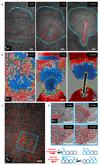

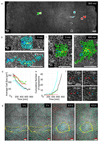

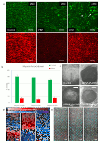


References
-
- Stern CD. Gastrulation, from cells to embryo′s. Cold Spring Harbor Laboratory Press; New York: 2004.
-
- Weinberger C, Brick I. Primary hypoblast development in the chick II. The role of cell division. Wilhelm Roux' Arch. Entwicklungsmechan.Org. 1982;191:127–133. - PubMed
-
- Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ. 2008;50:755–766. - PubMed
-
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G015082/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/R/00001603/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 094131/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- G0900740/MRC_/Medical Research Council/United Kingdom
- BBS/E/D/20320000/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 094131/WT_/Wellcome Trust/United Kingdom
- BB/E011276/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/K001744/1/MRC_/Medical Research Council/United Kingdom
- 094182/WT_/Wellcome Trust/United Kingdom
- BB/J004243/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

